Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2019-08-21 , DOI: 10.1016/j.jinorgbio.2019.110804
Giset Y Sánchez Delgado 1 , Diego Paschoal 2 , Marcone A L de Oliveira 3 , Hélio F Dos Santos 1
|
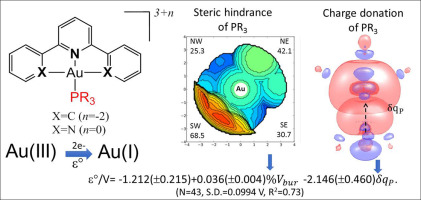
The choice of the auxiliary ligand in Au(III) complexes is of paramount importance in tuning their reactivity and biological activity. Tertiary phosphines are one of the most used auxiliary ligands in gold compounds, due to their stereo-electronic properties that confer stability and lipophilicity to these metallodrugs. The redox stability of [Au(III)(C^N^C)PR3]+ (A) (C^N^C = 2,6-diphenylpyridine) and [Au(III)(N^N^N)PR3]3+ (N^N^N = 2,2′:6′,2″-terpyridine) (B) complexes (where R is the phosphine substituent groups with different steric and electronic properties) was herein investigated for a set of 41 phosphines, using the predicted standard reduction potential (εo) for Au(III)/Au(I) electrochemical system as reference. For the complexes A, εo spread over 829 mV and all values were negative, whereas for the complexes B εo were positive and covered a narrower range of 507 mV. The phosphines with high buried volume (%Vbur ≥ 32%) decrease the complex stability despite being strong σ-donors. Both steric and electronic properties were used as molecular descriptors to build quantitative structure-property relationships (QSPR), which showed that the %Vbur plays the major role on the redox stability of the studied Au(III) complexes. For complexes B where the phosphine affects both Au(III) and Au(I) forms, the steric impact is more pronounced on the Au(I) reduced species. The electron-donating ability of phosphines is also important and plays a greater role on the redox stability of complexes B than complexes A. These outcomes are certainly useful to predict the redox stability of Au(III) complexes which, in turn, should affect their chemical reactivity against biological targets.
中文翻译:

水溶液中[Au(III)(X ^ N ^ X)PR3]配合物(X = C或N)的结构和氧化还原稳定性:膦辅助配体的作用。
Au(III)配合物中辅助配体的选择对于调节其反应性和生物活性至关重要。叔膦是金化合物中最常用的辅助配体之一,因为它们的立体电子性质赋予这些金属药物以稳定性和亲脂性。[Au(III)(C ^ N ^ C)PR 3 ] +(A)(C ^ N ^ C = 2,6-二苯基吡啶)和[Au(III)(N ^ N ^ N)PR 3 ]本文研究了3+(N ^ N ^ N = 2,2':6',2''-吡啶)(B)配合物(其中R是具有不同空间和电子特性的膦取代基)的配合物41次膦,使用预测的标准还原电势(ε Ò)作为Au(III)/ Au(I)电化学体系的参考。对于配合物A,ε ö分布在829毫伏和所有的值均为阴性,而对于络合物乙ε ö阳性,覆盖507 mV的更窄的范围。具有高埋入量(%V bur≥32 %)的膦尽管具有强大的σ供体,但仍降低了复合物的稳定性。空间和电子性质均用作分子描述符,以建立定量的结构-性质关系(QSPR),这表明%V bur在研究的Au(III)配合物的氧化还原稳定性中起主要作用。对于其中膦同时影响Au(III)和Au(I)形式的配合物B,空间影响在Au(I)还原物种上更为明显。膦的给电子能力也很重要,并且与配合物A相比,对配合物B的氧化还原稳定性起着更大的作用。这些结果对于预测Au(III)配合物的氧化还原稳定性无疑是有用的,反过来,Au(III)配合物也会影响它们的氧化还原稳定性。对生物学目标的化学反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号