当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics and Mechanism of Catalytic Oxidation of 5-Methylfurfural to 2,5-Furandicarboxylic Acid with Co/Mn/Br Catalyst
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-08-30 , DOI: 10.1021/acs.iecr.9b03573 Heng Ban 1 , Shuaibo Chen 1 , Youdi Zhang 1 , Youwei Cheng 1, 2 , Lijun Wang 1 , Xi Li 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-08-30 , DOI: 10.1021/acs.iecr.9b03573 Heng Ban 1 , Shuaibo Chen 1 , Youdi Zhang 1 , Youwei Cheng 1, 2 , Lijun Wang 1 , Xi Li 1
Affiliation
|
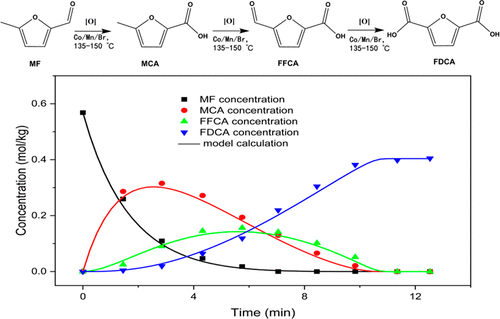
2,5-Furandicarboxylic acid (FDCA), a versatile platform chemical, can be widely used for synthesizing various polymers and has the potential to replace terephthalic acid in the green polymer industry. To replace part of the relatively expensive and chemically unstable 5-hydroxymethylfurfural (HMF), 5-methylfurfural (MF) was used as the starting material for preparing FDCA. The aerobic oxidation of MF to FDCA over the Co/Mn/Br catalyst system was investigated in both batch and semicontinuous reactors with acetic acid as solvent. On the basis of free-radical chain reaction mechanism, a fractional kinetic model was developed to describe the oxidation process of MF to FDCA and the relevant model parameters were determined by data fitting using the least-squares method. Under the same reaction conditions, the FDCA yield of 73.5% and purity of 99.5% were achieved, lower than those of HMF oxidation to FDCA. The influence of stirring rates was evaluated to eliminate the liquid–gas transfer resistance. Furthermore, the effects of substrate concentration, reaction temperature, and catalyst concentration on the reaction rate were investigated systematically. Finally, the developed fractional kinetic model was proved to be reliable for predicting the product distribution satisfactorily by the semicontinuous experiments. The kinetic data and reactor model play an essential role in the optimization for industrially producing FDCA.
中文翻译:

Co / Mn / Br催化剂催化5-甲基糠醛催化氧化为2,5-呋喃二甲酸的动力学及机理
2,5-呋喃二甲酸(FDCA)是一种通用的平台化学品,可广泛用于合成各种聚合物,在绿色聚合物工业中具有取代对苯二甲酸的潜力。为了代替部分相对昂贵且化学上不稳定的5-羟甲基糠醛(HMF),使用5-甲基糠醛(MF)作为制备FDCA的原料。在分批和半连续反应器中,以乙酸为溶剂,研究了Co / Mn / Br催化剂体系上MF的有氧氧化为FDCA。在自由基链反应机理的基础上,建立了分数动力学模型,描述了MF氧化为FDCA的过程,并通过最小二乘法对数据进行了拟合,确定了相关的模型参数。在相同的反应条件下,FDCA的收率为73.5%,纯度为99。达到5%,低于HMF氧化为FDCA的氧化率。评估了搅拌速率的影响,以消除液-气传递阻力。此外,系统地研究了底物浓度,反应温度和催化剂浓度对反应速率的影响。最后,通过半连续实验证明了所建立的分数动力学模型对于令人满意地预测产物分布是可靠的。动力学数据和反应器模型在工业生产FDCA的优化中起着至关重要的作用。考察了催化剂浓度对反应速率的影响。最后,通过半连续实验证明了所建立的分数动力学模型对于令人满意地预测产物分布是可靠的。动力学数据和反应器模型在工业生产FDCA的优化中起着至关重要的作用。考察了催化剂浓度对反应速率的影响。最后,通过半连续实验证明了所建立的分数动力学模型对于令人满意地预测产物分布是可靠的。动力学数据和反应器模型在工业生产FDCA的优化中起着至关重要的作用。
更新日期:2019-08-31
中文翻译:

Co / Mn / Br催化剂催化5-甲基糠醛催化氧化为2,5-呋喃二甲酸的动力学及机理
2,5-呋喃二甲酸(FDCA)是一种通用的平台化学品,可广泛用于合成各种聚合物,在绿色聚合物工业中具有取代对苯二甲酸的潜力。为了代替部分相对昂贵且化学上不稳定的5-羟甲基糠醛(HMF),使用5-甲基糠醛(MF)作为制备FDCA的原料。在分批和半连续反应器中,以乙酸为溶剂,研究了Co / Mn / Br催化剂体系上MF的有氧氧化为FDCA。在自由基链反应机理的基础上,建立了分数动力学模型,描述了MF氧化为FDCA的过程,并通过最小二乘法对数据进行了拟合,确定了相关的模型参数。在相同的反应条件下,FDCA的收率为73.5%,纯度为99。达到5%,低于HMF氧化为FDCA的氧化率。评估了搅拌速率的影响,以消除液-气传递阻力。此外,系统地研究了底物浓度,反应温度和催化剂浓度对反应速率的影响。最后,通过半连续实验证明了所建立的分数动力学模型对于令人满意地预测产物分布是可靠的。动力学数据和反应器模型在工业生产FDCA的优化中起着至关重要的作用。考察了催化剂浓度对反应速率的影响。最后,通过半连续实验证明了所建立的分数动力学模型对于令人满意地预测产物分布是可靠的。动力学数据和反应器模型在工业生产FDCA的优化中起着至关重要的作用。考察了催化剂浓度对反应速率的影响。最后,通过半连续实验证明了所建立的分数动力学模型对于令人满意地预测产物分布是可靠的。动力学数据和反应器模型在工业生产FDCA的优化中起着至关重要的作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号