当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Plasmonic Nanohybrid with High Photothermal Conversion Efficiency for Simultaneously Effective Antibacterial/Anticancer Photothermal Therapy
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2019-08-28 , DOI: 10.1021/acsabm.9b00521 Muhammad Rizwan Younis 1 , Rui Bing An 1 , Yun-Chao Yin 1 , Shouju Wang 2 , Deju Ye 1 , Xing-Hua Xia 1
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2019-08-28 , DOI: 10.1021/acsabm.9b00521 Muhammad Rizwan Younis 1 , Rui Bing An 1 , Yun-Chao Yin 1 , Shouju Wang 2 , Deju Ye 1 , Xing-Hua Xia 1
Affiliation
|
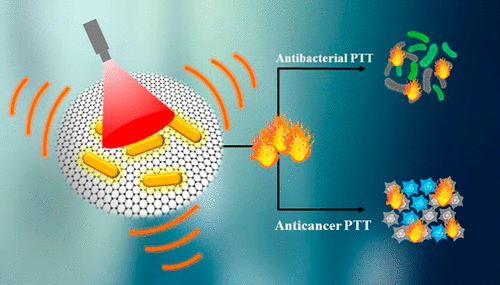
Plasmonic metal/semiconductor nanohybrids hold great promise in photocatalysis and biosensor development; however, their potential phototherapeutic applications are yet fully unexplored. On the other hand, the demand of high laser power density to induce antibacterial photothermal therapeutic effects greatly restricts the practical applicability of the previously developed photothermal nanoagents (PTAs) for anticancer photothermal therapy (PTT). Here, we develop a plasmonic nanohybrid by integrating plasmonic noble metal gold nanorods (AuNRs) with a two-dimensional graphene oxide (2-D GO), capable to perform photothermal ablation of both bacterial pathogens as well as tumor cells, respectively, under low power single near-infrared (NIR) laser activation. Owing to the synergistic plasmonic photothermal effect (PPTT) of dual plasmonic PTAs, the plasmonic AuNR/GO nanohybrid exhibits remarkably higher photothermal conversion efficiency (PCE, 72.59%) than either individual AuNRs or GO under low laser power density (300 mW), leading to enhanced antibacterial/anticancer PTT. In addition, the synergistic plasmonic antibacterial/anticancer PTT induced by the plasmonic nanohybrid is also far superior to individual PTAs (AuNRs or GO), whereas the flow cytometric analysis of heat shock proteins (HSP 70) clearly dictates that the substantial killing of bacterial pathogens/tumor cells is solely due to the synergistic PPTT. Thus, the plasmonic AuNR/GO nanohybrid is a standalone PTA to perform simultaneous antibacterial/anticancer PTT under low power NIR laser activation for only 5 min, without any systemic side effects. The present study provides a clear demonstration about the potential therapeutic impact of plasmonic nanohybrids and thus will surely pave the way to design other hybrid nanoagents with enhanced PCE and integrate them with chemotherapeutic agents, leading to dual-modal chemo-/photothermal antibacterial/anticancer therapy under low power single laser excitation for a short duration.
中文翻译:

具有高光热转换效率的等离子纳米杂化物,用于同时有效的抗菌/抗癌光热治疗
等离激元金属/半导体纳米杂化物在光催化和生物传感器开发中具有很大的应用前景;然而,它们潜在的光疗应用尚未完全探索。另一方面,高激光功率密度诱导抗菌光热治疗效果的需求极大地限制了先前开发的光热纳米剂(PTA)在抗癌光热治疗(PTT)中的实际应用。在这里,我们通过将等离子贵金属金纳米棒 (AuNR) 与二维氧化石墨烯 (2-D GO) 相结合,开发了一种等离子纳米杂化物,能够分别在低温下对细菌病原体和肿瘤细胞进行光热消融。功率单近红外 (NIR) 激光激活。由于双等离子体PTA的协同等离子体光热效应(PPTT),在低激光功率密度(300 mW)下,等离子 AuNR/GO 纳米混合物的光热转换效率(PCE,72.59%)明显高于单个 AuNR 或 GO,从而增强了抗菌/抗癌 PTT。此外,由等离子纳米混合物诱导的协同等离子抗菌/抗癌 PTT 也远远优于单个 PTA(AuNR 或 GO),而热休克蛋白(HSP 70)的流式细胞术分析清楚地表明,大量杀死细菌病原体/肿瘤细胞完全是由于协同PPTT。因此,等离子 AuNR/GO 纳米混合物是一种独立的 PTA,可在低功率 NIR 激光激活下仅 5 分钟同时进行抗菌/抗癌 PTT,没有任何全身副作用。
更新日期:2019-08-29
中文翻译:

具有高光热转换效率的等离子纳米杂化物,用于同时有效的抗菌/抗癌光热治疗
等离激元金属/半导体纳米杂化物在光催化和生物传感器开发中具有很大的应用前景;然而,它们潜在的光疗应用尚未完全探索。另一方面,高激光功率密度诱导抗菌光热治疗效果的需求极大地限制了先前开发的光热纳米剂(PTA)在抗癌光热治疗(PTT)中的实际应用。在这里,我们通过将等离子贵金属金纳米棒 (AuNR) 与二维氧化石墨烯 (2-D GO) 相结合,开发了一种等离子纳米杂化物,能够分别在低温下对细菌病原体和肿瘤细胞进行光热消融。功率单近红外 (NIR) 激光激活。由于双等离子体PTA的协同等离子体光热效应(PPTT),在低激光功率密度(300 mW)下,等离子 AuNR/GO 纳米混合物的光热转换效率(PCE,72.59%)明显高于单个 AuNR 或 GO,从而增强了抗菌/抗癌 PTT。此外,由等离子纳米混合物诱导的协同等离子抗菌/抗癌 PTT 也远远优于单个 PTA(AuNR 或 GO),而热休克蛋白(HSP 70)的流式细胞术分析清楚地表明,大量杀死细菌病原体/肿瘤细胞完全是由于协同PPTT。因此,等离子 AuNR/GO 纳米混合物是一种独立的 PTA,可在低功率 NIR 激光激活下仅 5 分钟同时进行抗菌/抗癌 PTT,没有任何全身副作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号