当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering an Alcohol Dehydrogenase for Balancing Kinetics in NADPH Regeneration with 1,4-Butanediol as a Cosubstrate
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-08-19 00:00:00 , DOI: 10.1021/acssuschemeng.9b03879 Guochao Xu 1 , Cheng Zhu 1 , Aitao Li 2 , Yan Ni 3 , Ruizhi Han 1 , Jieyu Zhou 1 , Ye Ni 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-08-19 00:00:00 , DOI: 10.1021/acssuschemeng.9b03879 Guochao Xu 1 , Cheng Zhu 1 , Aitao Li 2 , Yan Ni 3 , Ruizhi Han 1 , Jieyu Zhou 1 , Ye Ni 1
Affiliation
|
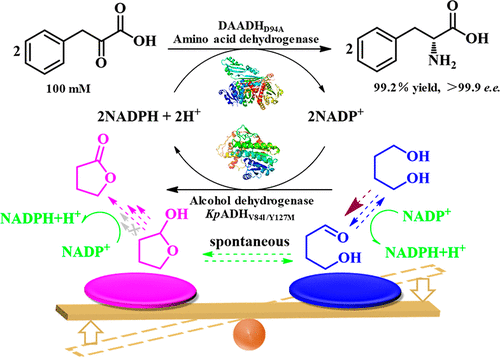
Cofactor regeneration using diols as “smart cosubstrates” is one of the most promising approaches, due to the thermodynamic preference and 0.5-equiv requirement. In order to establish an efficient NADPH regeneration system with 1,4-butanediol (1,4-BD), a NADP+-dependent alcohol dehydrogenase from Kluyveromyces polysporus (KpADH) was engineered to solve the kinetic imbalance. Several hotspots were identified through molecular dynamic simulation and subjected to saturation and combinatorial mutagenesis. Variant KpADHV84I/Y127M exhibited a lower KM of 15.1 mM and a higher kcat of 30.1 min–1 than WTKpADH. The oxidation of 1,4-BD to 4-hydroxybutanal was found to be the rate-limiting step, for which the kcat/KM value of double mutant KpADHV84I/Y127M was 2.00 min–1·mM–1, 11.6-fold higher than that of WTKpADH. KpADHV84I/Y127M preferred diols with a longer chain length (C5–C6). The ratio of kcat/KM toward 2-hydroxytetrahydrofuran (2-HTHF), in comparison to 1,4-BD, in KpADHV84I/Y127M was dramatically reduced by almost 100-fold compared to WTKpADH, which was advantageous for NADPH regeneration. As much as 100 mM phenylpyruvic acid could be reduced into d-phenylalanine with 99.2% conversion in 6 h using merely 0.5 equiv of 1,4-BD. Both the improved catalytic efficiency toward 1,4-BD and the balanced kcat/KM between 1,4-BD and 2-HTHF contributed to the higher NADPH regeneration efficiency. This study provides guidance for engineering alcohol dehydrogenases for cosubstrate specificity toward diols and its application in NADPH regeneration for the preparation of chiral compounds of pharmaceutical relevance.
中文翻译:

以1,4-丁二醇为辅助底物,设计一种酒精脱氢酶以平衡NADPH再生动力学。
由于热力学偏好和0.5当量的要求,使用二醇作为“智能共底物”的辅因子再生是最有前途的方法之一。为了建立具有1,4-丁二醇(1,4-BD)的高效NADPH再生系统,设计了多孢克鲁维酵母(Kp ADH)的NADP +依赖性醇脱氢酶,以解决动力学失衡问题。通过分子动力学模拟确定了几个热点,并进行了饱和和组合诱变。变体的Kp ADH V84I / Y127M表现出较低ķ中号15.1毫和较高ķ猫的30.1分钟-1比WT Kp ADH。1,4-BD与4-羟基丁醛氧化被认为是限速步骤,为此,ķ猫/ ķ中号双突变体的值的Kp ADH V84I / Y127M是2.00分钟-1 ·毫米-1,11.6 -是WT Kp ADH的三倍。Kp ADH V84I / Y127M优选链长较长的二醇(C5-C6)。在Kp ADH V84I / Y127M中,与1,4-BD相比,k cat / K M与2-羟基四氢呋喃(2-HTHF)的比率与WT Kp ADH相比,其显着降低了近100倍,这对NADPH再生非常有利。仅使用0.5当量的1,4-BD,在6小时内,可以将多达100 mM的苯丙酮酸还原为d-苯丙氨酸,转化率为99.2%。对1,4-BD的提高的催化效率和1,4-BD与2-HTHF之间的平衡k cat / K M均有助于更高的NADPH再生效率。这项研究为工程醇脱氢酶的共底物对二醇的特异性及其在NADPH再生中的应用提供了指导,以制备具有药物相关性的手性化合物。
更新日期:2019-08-19
中文翻译:

以1,4-丁二醇为辅助底物,设计一种酒精脱氢酶以平衡NADPH再生动力学。
由于热力学偏好和0.5当量的要求,使用二醇作为“智能共底物”的辅因子再生是最有前途的方法之一。为了建立具有1,4-丁二醇(1,4-BD)的高效NADPH再生系统,设计了多孢克鲁维酵母(Kp ADH)的NADP +依赖性醇脱氢酶,以解决动力学失衡问题。通过分子动力学模拟确定了几个热点,并进行了饱和和组合诱变。变体的Kp ADH V84I / Y127M表现出较低ķ中号15.1毫和较高ķ猫的30.1分钟-1比WT Kp ADH。1,4-BD与4-羟基丁醛氧化被认为是限速步骤,为此,ķ猫/ ķ中号双突变体的值的Kp ADH V84I / Y127M是2.00分钟-1 ·毫米-1,11.6 -是WT Kp ADH的三倍。Kp ADH V84I / Y127M优选链长较长的二醇(C5-C6)。在Kp ADH V84I / Y127M中,与1,4-BD相比,k cat / K M与2-羟基四氢呋喃(2-HTHF)的比率与WT Kp ADH相比,其显着降低了近100倍,这对NADPH再生非常有利。仅使用0.5当量的1,4-BD,在6小时内,可以将多达100 mM的苯丙酮酸还原为d-苯丙氨酸,转化率为99.2%。对1,4-BD的提高的催化效率和1,4-BD与2-HTHF之间的平衡k cat / K M均有助于更高的NADPH再生效率。这项研究为工程醇脱氢酶的共底物对二醇的特异性及其在NADPH再生中的应用提供了指导,以制备具有药物相关性的手性化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号