Nature Chemistry ( IF 19.2 ) Pub Date : 2019-08-19 , DOI: 10.1038/s41557-019-0306-x Erli Lu 1 , Benjamin E Atkinson 1 , Ashley J Wooles 1 , Josef T Boronski 1 , Laurence R Doyle 1 , Floriana Tuna 1 , Jonathan D Cryer 1 , Philip J Cobb 1 , Inigo J Vitorica-Yrezabal 1 , George F S Whitehead 1 , Nikolas Kaltsoyannis 1 , Stephen T Liddle 1
|
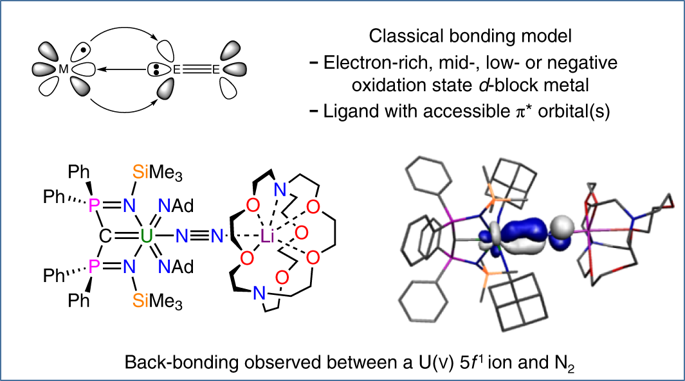
A fundamental bonding model in coordination and organometallic chemistry is the synergic, donor–acceptor interaction between a metal and a neutral π-acceptor ligand, in which the ligand σ donates to the metal, which π back-bonds to the ligand. This interaction typically involves a metal with an electron-rich, mid-, low- or even negative oxidation state and a ligand with a π* orbital. Here, we report that treatment of a uranium–carbene complex with an organoazide produces a uranium(v)–bis(imido)–dinitrogen complex, stabilized by a lithium counterion. This complex, which was isolated in a crystalline form, involves an electron-poor, high-oxidation-state uranium(v) 5f1 ion that is π back-bonded to the poor π-acceptor ligand dinitrogen. We propose that this is made possible by a combination of cooperative heterobimetallic uranium–lithium effects and the presence of suitable ancillary ligands that render the uranium ion unusually electron rich. This electron-poor back-bonding could have implications for the field of dinitrogen activation.
中文翻译:

贫电子,高氧化态金属与铀(V)-二氮络合物中较差的π受体配体之间的反向键合。
配位和有机金属化学中的基本键合模型是金属与中性π-受体配体之间的协同施主-受体相互作用,其中配体σ贡献给金属,而π反向键合到配体上。这种相互作用通常涉及具有富电子,中,低或什至负氧化态的金属和具有π *轨道的配体。在这里,我们报道用有机叠氮化物处理铀-卡宾络合物会产生铀(v)-双(亚氨基)-二氮络合物,并由锂抗衡离子稳定。以晶体形式分离出的该络合物涉及电子贫乏的高氧化态铀(v)5 fπ背键键合至不良π受体配体二氮的1个离子。我们认为,这是通过协同异双金属铀-锂效应以及合适的辅助配体(使铀离子异常富电子)的结合而实现的。这种电子贫乏的背键可能对二氮活化领域有影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号