当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First total synthesis of rhuscholide A, glabralide B and denudalide
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-08-17 , DOI: 10.1016/j.tetlet.2019.151059 Tian-Ze Li , Chang-An Geng , Ji-Jun Chen
中文翻译:

首次全合成鼠李糖苷A,glabralide B和denudalide
更新日期:2019-08-17
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-08-17 , DOI: 10.1016/j.tetlet.2019.151059 Tian-Ze Li , Chang-An Geng , Ji-Jun Chen
|
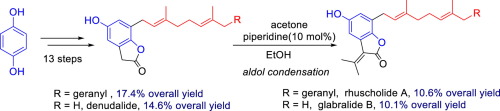
The first total synthesis of rhuscholide A, a benzofuran lactone possessing anti-HIV-1 activity, had been accomplished in 14 linear steps with 10.6% overall yield. In this synthesis, base-mediated phenol ortho-alkylation and piperidine promoted aldol condensation were exploited as key steps. The synthesis was flexible and allowed for the convenient preparation of two analogous natural products glabralide B and denudalide.
中文翻译:

首次全合成鼠李糖苷A,glabralide B和denudalide
具有14个线性步骤,以14.6%的总收率完成了具有抗HIV-1活性的苯并呋喃内酯Rhuscholide A的第一个全合成。在该合成中,将碱介导的苯酚正烷基化和哌啶促进的醇醛缩合用作关键步骤。该合成是灵活的,并且允许方便地制备两种类似的天然产物glabralide B和denudalide。






























 京公网安备 11010802027423号
京公网安备 11010802027423号