Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydration and Secondary Ozonide of the Criegee Intermediate of Sabinene
ACS Omega ( IF 3.7 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acsomega.7b02002 Mansour H. Almatarneh 1, 2 , Ismael A. Elayan 1 , Mohammednoor Altarawneh 3 , Joshua W. Hollett 4
ACS Omega ( IF 3.7 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acsomega.7b02002 Mansour H. Almatarneh 1, 2 , Ismael A. Elayan 1 , Mohammednoor Altarawneh 3 , Joshua W. Hollett 4
Affiliation
|
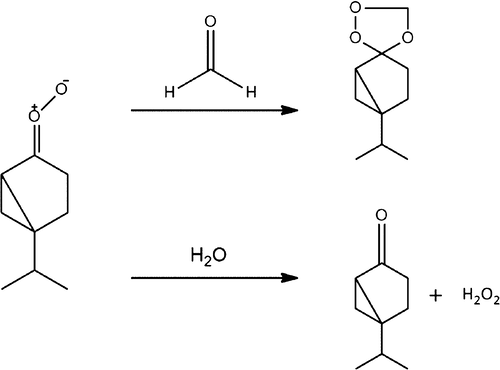
A computational study of the formation of secondary ozonide (SOZ) from the Criegee intermediates (CIs) of sabinene, including hydration reactions with H2O and 2H2O, was performed. All of the geometries were optimized at the B3LYP and M06-2X with several basis sets. Further single-point energy calculation at the CCSD(T) was performed. Two major pathways of SOZ formation suggest that it is mainly formed from the sabinene CI and formaldehyde rather than sabina ketone and formaldehyde-oxide. However, in both pathways, the activation energies are within a range of ±5 kJ mol–1. Furthermore, the hydration reactions of the anti-CI with H2O and 2H2O showed that the role of the second water molecule is a mediator (catalyst) in this reaction. The dimer hydration reaction has lower activation energies than the monomer by 60 and 69 kJ mol–1, at the M06-2X/6-31G(d) and CCSD(T)+CF levels of the theory, respectively. A novel water-mediated vinyl hydroperoxide (VHP) channel from both the monomer and dimer has been investigated. The results indicate that the direct nonmediated VHP formation and dissociation is interestingly more possible than the water-mediated VHP. The density functional theory calculations show that the monomer is faster than the dimer by roughly 22 kJ mol–1. Further, the infrared spectrum of sabina ketone was calculated at B3LYP/6-311+G(2d,p); the calculated carbonyl stretching of 1727 cm–1 is in agreement with the experimental range of 1700–1800 cm–1.
中文翻译:

Sabinene的Criegee中间体的水合和二次臭氧
进行了从sa烯的Criegee中间体(CIs)形成次级臭氧化物(SOZ)的计算研究,包括与H 2 O和2H 2 O的水合反应。在B3LYP和M06-2X上,使用多个基本集对所有几何进行了优化。在CCSD(T)上进行了进一步的单点能量计算。SOZ形成的两个主要途径表明,它主要是由Sabinene CI和甲醛形成的,而不是由Sabina酮和甲醛氧化物形成的。但是,在这两种途径中,活化能都在±5 kJ mol –1的范围内。此外,抗CI与H 2 O和2H 2的水合反应O表明第二个水分子在该反应中的作用是介质(催化剂)。在该理论的M06-2X / 6-31G(d)和CCSD(T)+ CF浓度下,二聚体水合反应的活化能比单体分别低60和69 kJ mol –1。研究了来自单体和二聚体的新型水介导的乙烯基氢过氧化物(VHP)通道。结果表明,有趣的是,与水介导的VHP相比,直接的非介导VHP的形成和解离更有可能。密度泛函理论计算表明,单体比二聚体快约22 kJ mol –1。另外,计算出Sabina酮的红外光谱为B3LYP / 6-311 + G(2d,p)。计算得出的羰基拉伸度为1727 cm –1与1700–1800 cm –1的实验范围一致。
更新日期:2018-02-28
中文翻译:

Sabinene的Criegee中间体的水合和二次臭氧
进行了从sa烯的Criegee中间体(CIs)形成次级臭氧化物(SOZ)的计算研究,包括与H 2 O和2H 2 O的水合反应。在B3LYP和M06-2X上,使用多个基本集对所有几何进行了优化。在CCSD(T)上进行了进一步的单点能量计算。SOZ形成的两个主要途径表明,它主要是由Sabinene CI和甲醛形成的,而不是由Sabina酮和甲醛氧化物形成的。但是,在这两种途径中,活化能都在±5 kJ mol –1的范围内。此外,抗CI与H 2 O和2H 2的水合反应O表明第二个水分子在该反应中的作用是介质(催化剂)。在该理论的M06-2X / 6-31G(d)和CCSD(T)+ CF浓度下,二聚体水合反应的活化能比单体分别低60和69 kJ mol –1。研究了来自单体和二聚体的新型水介导的乙烯基氢过氧化物(VHP)通道。结果表明,有趣的是,与水介导的VHP相比,直接的非介导VHP的形成和解离更有可能。密度泛函理论计算表明,单体比二聚体快约22 kJ mol –1。另外,计算出Sabina酮的红外光谱为B3LYP / 6-311 + G(2d,p)。计算得出的羰基拉伸度为1727 cm –1与1700–1800 cm –1的实验范围一致。















































 京公网安备 11010802027423号
京公网安备 11010802027423号