当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis and Anti-Cancer Activity of All Known Communesin Alkaloids and Related Derivatives
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-08-17 , DOI: 10.1021/jacs.9b07397 Matthew M Pompeo 1 , Jaime H Cheah 2 , Mohammad Movassaghi 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-08-17 , DOI: 10.1021/jacs.9b07397 Matthew M Pompeo 1 , Jaime H Cheah 2 , Mohammad Movassaghi 1
Affiliation
|
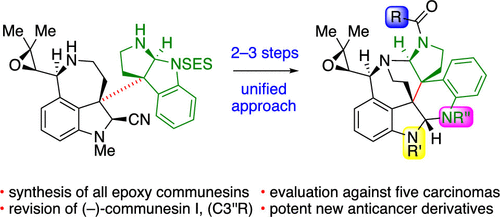
A unified enantioselective total synthesis and anticancer evaluation of all known epoxide-containing communesin alkaloids and related derivatives is described. Our synthesis is predicated on the convergent and modular diazene-directed assembly of two complex fragments to secure the critical C3a–C3a′ linkage followed by a guided biomimetic aminal reorganization to deliver the heptacyclic core of these alkaloids. Concise enantioselective syntheses of the fragments were devised, with highlights including the application of a rationally designed sulfinamide chiral auxiliary, an efficient calcium trifluoromethanesulfonate promoted intramolecular amination, and a diastereoselective epoxidation that simultaneously converts the new chiral auxiliary to a versatile amine protective group. The modularity of our convergent approach enabled the rapid synthesis of all epoxide-containing members of the communesin family from a single heterodimeric intermediate, including the first total synthesis of communesins C–E, and G–I, and facilitated our stereochemical revision of (−)-communesin I, the most recently isolated communesin alkaloid. Furthermore, the generality of our biogenetically inspired heterodimer rearrangement was demonstrated in a guided synthesis of a communesin derivative with an unnatural topology. Finally, we report the first comparative analysis of the anticancer activities of all naturally occurring communesin alkaloids A–I and eight complex derivatives against five human cancer cell lines. From these data, we have identified (−)-communesin B as the most potent natural communesin and discovered that derivatives with N8′-sulfonamide substitution exhibit up to a 10-fold increase in potency over the natural alkaloids.
中文翻译:

所有已知 Communesin 生物碱及相关衍生物的全合成和抗癌活性
描述了所有已知的含环氧化物的 communesin 生物碱和相关衍生物的统一对映选择性全合成和抗癌评价。我们的合成基于两个复杂片段的会聚和模块化二氮烯定向组装,以确保关键的 C3a-C3a' 连接,然后进行引导仿生氨基重组,以提供这些生物碱的七环核心。设计了片段的简洁对映选择性合成,重点包括应用合理设计的亚磺酰胺手性助剂、高效的三氟甲磺酸钙促进分子内胺化,以及非对映选择性环氧化,同时将新的手性助剂转化为多功能胺保护基团。我们的聚合方法的模块化使得能够从单个异二聚体中间体快速合成 communesin 家族的所有含环氧化物成员,包括首次全合成 communesins C-E 和 G-I,并促进了我们对 (− )-communesin I,最新分离的 communesin 生物碱。此外,我们的生物遗传学启发的异二聚体重排的普遍性在具有非自然拓扑结构的 communesin 衍生物的引导合成中得到了证明。最后,我们报告了所有天然存在的 communesin 生物碱 A-I 和八种复杂衍生物针对五种人类癌细胞系的抗癌活性的首次比较分析。根据这些数据,我们确定 (−)-communesin B 是最有效的天然 communesin,并发现具有 N8'-磺酰胺取代的衍生物的效力比天然生物碱高出 10 倍。
更新日期:2019-08-17
中文翻译:

所有已知 Communesin 生物碱及相关衍生物的全合成和抗癌活性
描述了所有已知的含环氧化物的 communesin 生物碱和相关衍生物的统一对映选择性全合成和抗癌评价。我们的合成基于两个复杂片段的会聚和模块化二氮烯定向组装,以确保关键的 C3a-C3a' 连接,然后进行引导仿生氨基重组,以提供这些生物碱的七环核心。设计了片段的简洁对映选择性合成,重点包括应用合理设计的亚磺酰胺手性助剂、高效的三氟甲磺酸钙促进分子内胺化,以及非对映选择性环氧化,同时将新的手性助剂转化为多功能胺保护基团。我们的聚合方法的模块化使得能够从单个异二聚体中间体快速合成 communesin 家族的所有含环氧化物成员,包括首次全合成 communesins C-E 和 G-I,并促进了我们对 (− )-communesin I,最新分离的 communesin 生物碱。此外,我们的生物遗传学启发的异二聚体重排的普遍性在具有非自然拓扑结构的 communesin 衍生物的引导合成中得到了证明。最后,我们报告了所有天然存在的 communesin 生物碱 A-I 和八种复杂衍生物针对五种人类癌细胞系的抗癌活性的首次比较分析。根据这些数据,我们确定 (−)-communesin B 是最有效的天然 communesin,并发现具有 N8'-磺酰胺取代的衍生物的效力比天然生物碱高出 10 倍。































 京公网安备 11010802027423号
京公网安备 11010802027423号