当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of Atomic Hydrogen with the Cu2O(100) and (111) Surfaces
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-08-28 , DOI: 10.1021/acs.jpcc.9b03888 Heloise Tissot , Chunlei Wang , Joakim Halldin Stenlid 1 , Mohammad Panahi 2, 3 , Sarp Kaya 2, 3 , Markus Soldemo , Milad Ghadami Yazdi , Tore Brinck , Jonas Weissenrieder
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-08-28 , DOI: 10.1021/acs.jpcc.9b03888 Heloise Tissot , Chunlei Wang , Joakim Halldin Stenlid 1 , Mohammad Panahi 2, 3 , Sarp Kaya 2, 3 , Markus Soldemo , Milad Ghadami Yazdi , Tore Brinck , Jonas Weissenrieder
Affiliation
|
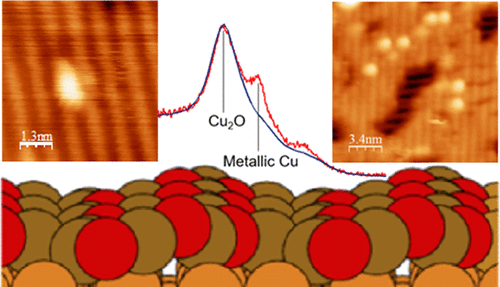
Reduction of Cu2O by hydrogen is a common preparation step for heterogeneous catalysts; however, a detailed understanding of the atomic reaction pathways is still lacking. Here, we investigate the interaction of atomic hydrogen with the Cu2O(100):(3,0;1,1) and Cu2O(111):(√3 × √3)R30° surfaces using scanning tunneling microscopy (STM), low-energy electron diffraction, temperature-programmed desorption (TPD), and X-ray photoelectron spectroscopy (XPS). The experimental results are compared to density functional theory simulations. At 300 K, we identify the most favorable adsorption site on the Cu2O(100) surface: hydrogen atoms bind to an oxygen site located at the base of the atomic rows intrinsic to the (3,0;1,1) surface. The resulting hydroxyl group subsequently migrates to a nearby Cu trimer site. TPD analysis identifies H2 as the principal desorption product. These observations imply that H2 is formed through a disproportionation reaction of surface hydroxyl groups. The interaction of H with the (111) surface is more complex, including coordination to both Cu+ and OCUS sites. STM and XPS analyses reveal the formation of metallic copper clusters on the Cu2O surfaces after cycles of hydrogen exposure and annealing. The interaction of the Cu clusters with the substrate is notably different for the two surface terminations studied: after annealing, the Cu clusters coalesce on the (100) termination, and the (3,0;1,1) reconstruction is partially recovered. Clusters formed on the (111) surface are less prone to coalescence, and the (√3 × √3)R30° reconstruction was not recovered by heat treatment, indicating a weaker Cu cluster to support interaction on the (100) surface.
中文翻译:

原子氢与Cu2O(100)和(111)表面的相互作用
氢还原Cu 2 O是非均相催化剂的常见制备步骤;但是,仍然缺乏对原子反应途径的详细了解。在这里,我们使用扫描隧道显微镜研究原子氢与Cu 2 O(100):( 3,0; 1,1)和Cu 2 O(111):(√3×√3)R 30°表面的相互作用(STM),低能电子衍射,程序升温解吸(TPD)和X射线光电子能谱(XPS)。将实验结果与密度泛函理论仿真进行了比较。在300 K时,我们确定了Cu 2上最有利的吸附位点O(100)表面:氢原子与位于(3,0; 1,1)表面固有的原子行底部的氧位点结合。所得的羟基随后迁移至附近的Cu三聚体位点。TPD分析确定H 2是主要的解吸产物。这些观察结果暗示H 2是通过表面羟基的歧化反应形成的。H与(111)表面的相互作用更复杂,包括与Cu +和O CUS位点的配位。STM和XPS分析表明在Cu 2上形成了金属铜簇经过氢暴露和退火循环后的O表面。对于研究的两个表面终端,Cu团簇与基材的相互作用显着不同:退火后,Cu团簇在(100)终端上聚结,并且部分恢复了(3,0; 1,1)重建。在(111)表面上形成的团簇不易聚结,并且(√3×√3)R 30°重构未通过热处理恢复,表明较弱的Cu团簇可支持(100)表面上的相互作用。
更新日期:2019-08-29
中文翻译:

原子氢与Cu2O(100)和(111)表面的相互作用
氢还原Cu 2 O是非均相催化剂的常见制备步骤;但是,仍然缺乏对原子反应途径的详细了解。在这里,我们使用扫描隧道显微镜研究原子氢与Cu 2 O(100):( 3,0; 1,1)和Cu 2 O(111):(√3×√3)R 30°表面的相互作用(STM),低能电子衍射,程序升温解吸(TPD)和X射线光电子能谱(XPS)。将实验结果与密度泛函理论仿真进行了比较。在300 K时,我们确定了Cu 2上最有利的吸附位点O(100)表面:氢原子与位于(3,0; 1,1)表面固有的原子行底部的氧位点结合。所得的羟基随后迁移至附近的Cu三聚体位点。TPD分析确定H 2是主要的解吸产物。这些观察结果暗示H 2是通过表面羟基的歧化反应形成的。H与(111)表面的相互作用更复杂,包括与Cu +和O CUS位点的配位。STM和XPS分析表明在Cu 2上形成了金属铜簇经过氢暴露和退火循环后的O表面。对于研究的两个表面终端,Cu团簇与基材的相互作用显着不同:退火后,Cu团簇在(100)终端上聚结,并且部分恢复了(3,0; 1,1)重建。在(111)表面上形成的团簇不易聚结,并且(√3×√3)R 30°重构未通过热处理恢复,表明较弱的Cu团簇可支持(100)表面上的相互作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号