当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isomerically Pure Benzothiophene-Incorporated Acceptor: Achieving Improved Voc and Jsc of Nonfullerene Organic Solar Cells via End Group Manipulation
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-08-16 00:00:00 , DOI: 10.1021/acsami.9b08462
Shao-Ling Chang,Kai-En Hung,Fong-Yi Cao,Kuo-Hsiu Huang,Chain-Shu Hsu,Chuang-Yi Liao,Chia-Hao Lee,Yen-Ju Cheng
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-08-16 00:00:00 , DOI: 10.1021/acsami.9b08462
Shao-Ling Chang,Kai-En Hung,Fong-Yi Cao,Kuo-Hsiu Huang,Chain-Shu Hsu,Chuang-Yi Liao,Chia-Hao Lee,Yen-Ju Cheng
|
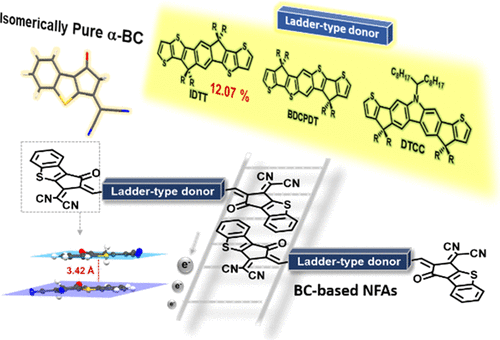
Benzene-based 1,1-dicyanomethylene-3-indanone (IC) derivatives have been widely utilized as the end-group to construct acceptor–donor–acceptor type nonfullerene acceptors (A–D–A type NFAs). The extension of the end-group conjugation of nonfullerene acceptors (NFAs) is a rational strategy to facilitate intermolecular stacking of the end-groups which are responsible for efficient electron transportation. A bicyclic benzothiophene-based end-group acceptor, 2-(3-oxo-2,3-dihydro-1H-benzo[b]cyclopenta[d]thiophen-1-ylidene)malononitrile, denoted as α-BC was designed and synthesized. The Knoevenagel condensation of the unsymmetrical 1,3-diketo-precursor with one equivalent of malononitrile selectively reacts with the keto group attached at the α-position of the thiophene unit, leading to the isomerically pure benzothiophene-fused α-BC. The well-defined α-BC with extended conjugation was condensed with three different ladder-type diformylated donors to form three new A–D–A NFAs named BDCPDT-BC, DTCC-BC, and ITBC, respectively. The corresponding IC-based BDCPDT-IC, DTCC-IC, and ITIC model compounds were also synthesized for comparison. The incorporation of the electron-rich benzothiophene unit in the end-group upshifts the lowest unoccupied molecular orbital energy levels of the NFAs, which beneficially enlarges the Voc values. On the other hand, the benzothiophene unit in α-BC not also imparts an optical transition in the shorter wavelengths around 340–400 nm for a better light harvesting ability but also promotes the antiparallel π–π stacking of the end-groups for efficient electron transport. The organic photovoltaic cell devices using a PBDB-T polymer and BC-based NFAs all showed the improved Voc and Jsc values. The BDCPDT-BC- and DTCC-BC-based devices exhibited a power conversion efficiency (PCE) of 10.82 and 10.74%, respectively, which outperformed the corresponding BDCPDT-IC-, and DTCC-IC-based devices (9.33 and 9.25%). More importantly, the ITBC-based device delivered the highest PCE of 12.07% with a Jsc of 19.90 mA/cm2, a Voc of 0.94 V, and an fill factor of 64.51%, representing a 14% improvement relative to the traditional ITIC-based device (10.05%).
中文翻译:

异构纯苯并噻吩的受体:通过端基处理实现改进的非富勒烯有机太阳能电池的V oc和J sc
基于苯的1,1-二氰基亚甲基-3-茚满酮(IC)衍生物已被广泛用作构建受体-供体-受体型非富勒烯受体(AD-A型NFA)的端基。非富勒烯受体(NFA)端基共轭的扩展是一种合理的策略,以促进负责高效电子传输的端基的分子间堆叠。基于双环苯并噻吩的端基受体2-(3-氧代-2,3-二氢-1 H-苯并[ b ]环戊[ d ]设计合成了噻吩-1-亚甲基丙二腈。不对称的1,3-二酮前体与一当量的丙二腈的Knoevenagel缩合选择性地与连接在噻吩单元α位置的酮基反应,生成异构纯的苯并噻吩稠合的α-BC。将定义明确的具有扩展共轭作用的α-BC与三个不同的梯型二甲酰化供体缩合,分别形成三个新的A–D–A NFA,分别命名为BDCPDT-BC,DTCC-BC和ITBC。还合成了相应的基于IC的BDCPDT-IC,DTCC-IC和ITIC模型化合物以进行比较。末端基团中富含电子的苯并噻吩单元的引入使NFA的最低未占据分子轨道能级升高,这有利于增大Voc值。另一方面,α-BC中的苯并噻吩单元不仅可以在340-400 nm左右的较短波长处产生光学跃迁,从而具有更好的光收集能力,而且还可以促进端基的反平行π-π堆积,从而实现高效电子运输。使用PBDB-T聚合物和基于BC的NFA的有机光伏电池装置均显示出改善的V oc和J sc值。基于BDCPDT-BC和DTCC-BC的设备的功率转换效率(PCE)分别为10.82和10.74%,超过相应的基于BDCPDT-IC和DTCC-IC的设备(9.33和9.25%) 。更重要的是,所述基于ITBC-装置递送的12.07%的最高PCE与Ĵ SC相对于传统的基于ITIC的设备(10.05%),它的输出电流为19.90 mA / cm 2,V oc为0.94 V,填充系数为64.51%,提高了14%。
更新日期:2019-08-16
中文翻译:

异构纯苯并噻吩的受体:通过端基处理实现改进的非富勒烯有机太阳能电池的V oc和J sc
基于苯的1,1-二氰基亚甲基-3-茚满酮(IC)衍生物已被广泛用作构建受体-供体-受体型非富勒烯受体(AD-A型NFA)的端基。非富勒烯受体(NFA)端基共轭的扩展是一种合理的策略,以促进负责高效电子传输的端基的分子间堆叠。基于双环苯并噻吩的端基受体2-(3-氧代-2,3-二氢-1 H-苯并[ b ]环戊[ d ]设计合成了噻吩-1-亚甲基丙二腈。不对称的1,3-二酮前体与一当量的丙二腈的Knoevenagel缩合选择性地与连接在噻吩单元α位置的酮基反应,生成异构纯的苯并噻吩稠合的α-BC。将定义明确的具有扩展共轭作用的α-BC与三个不同的梯型二甲酰化供体缩合,分别形成三个新的A–D–A NFA,分别命名为BDCPDT-BC,DTCC-BC和ITBC。还合成了相应的基于IC的BDCPDT-IC,DTCC-IC和ITIC模型化合物以进行比较。末端基团中富含电子的苯并噻吩单元的引入使NFA的最低未占据分子轨道能级升高,这有利于增大Voc值。另一方面,α-BC中的苯并噻吩单元不仅可以在340-400 nm左右的较短波长处产生光学跃迁,从而具有更好的光收集能力,而且还可以促进端基的反平行π-π堆积,从而实现高效电子运输。使用PBDB-T聚合物和基于BC的NFA的有机光伏电池装置均显示出改善的V oc和J sc值。基于BDCPDT-BC和DTCC-BC的设备的功率转换效率(PCE)分别为10.82和10.74%,超过相应的基于BDCPDT-IC和DTCC-IC的设备(9.33和9.25%) 。更重要的是,所述基于ITBC-装置递送的12.07%的最高PCE与Ĵ SC相对于传统的基于ITIC的设备(10.05%),它的输出电流为19.90 mA / cm 2,V oc为0.94 V,填充系数为64.51%,提高了14%。

































 京公网安备 11010802027423号
京公网安备 11010802027423号