当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Electrochemical Oxidation of Cu2S into CuO Nanowires as a Durable and Efficient Electrocatalyst for Oxygen Evolution Reaction
Chemistry of Materials ( IF 7.2 ) Pub Date : 2019-08-28 , DOI: 10.1021/acs.chemmater.9b02790 Yong Zuo 1, 2 , Yongpeng Liu 3 , Junshan Li 1, 2 , Ruifeng Du 1, 2 , Xu Han 4 , Ting Zhang 4 , Jordi Arbiol 4, 5 , Núria J. Divins 6 , Jordi Llorca 6 , Néstor Guijarro 3 , Kevin Sivula 3 , Andreu Cabot 1, 5
Chemistry of Materials ( IF 7.2 ) Pub Date : 2019-08-28 , DOI: 10.1021/acs.chemmater.9b02790 Yong Zuo 1, 2 , Yongpeng Liu 3 , Junshan Li 1, 2 , Ruifeng Du 1, 2 , Xu Han 4 , Ting Zhang 4 , Jordi Arbiol 4, 5 , Núria J. Divins 6 , Jordi Llorca 6 , Néstor Guijarro 3 , Kevin Sivula 3 , Andreu Cabot 1, 5
Affiliation
|
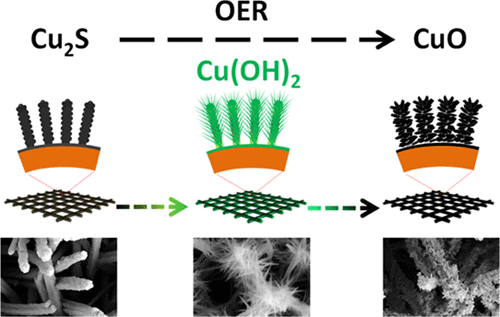
Development of cost-effective oxygen evolution catalysts is of capital importance for the deployment of large-scale energy-storage systems based on metal–air batteries and reversible fuel cells. In this direction, a wide range of materials have been explored, especially under more favorable alkaline conditions, and several metal chalcogenides have particularly demonstrated excellent performances. However, chalcogenides are thermodynamically less stable than the corresponding oxides and hydroxides under oxidizing potentials in alkaline media. Although this instability in some cases has prevented the application of chalcogenides as oxygen evolution catalysts and it has been disregarded in some others, we propose to use it in our favor to produce high-performance oxygen evolution catalysts. We characterize here the in situ chemical, structural, and morphological transformation during the oxygen evolution reaction (OER) in alkaline media of Cu2S into CuO nanowires, mediating the intermediate formation of Cu(OH)2. We also test their OER activity and stability under OER operation in alkaline media and compare them with the OER performance of Cu(OH)2 and CuO nanostructures directly grown on the surface of a copper mesh. We demonstrate here that CuO produced from in situ electrochemical oxidation of Cu2S displays an extraordinary electrocatalytic performance toward OER, well above that of CuO and Cu(OH)2 synthesized without this transformation.
中文翻译:

Cu2S原位电化学氧化成CuO纳米线作为一种持久有效的氧析出反应电催化剂
对于部署基于金属-空气电池和可逆燃料电池的大规模储能系统而言,开发具有成本效益的制氧催化剂至关重要。在这个方向上,已经探索了广泛的材料,特别是在更有利的碱性条件下,并且几种金属硫属化物特别表现出优异的性能。然而,在碱性介质中的氧化电势下,硫属化物的热力学稳定性低于相应的氧化物和氢氧化物。尽管这种不稳定性在某些情况下阻止了硫族化物用作制氧催化剂,而在另一些情况下却被忽略,但我们建议使用它来生产高性能的制氧催化剂。我们在这里描述原位化学,结构,2 S转变为CuO纳米线,介导Cu(OH)2的中间形成。我们还测试了它们在碱性介质中在OER下的OER活性和稳定性,并将它们与直接生长在铜网表面的Cu(OH)2和CuO纳米结构的OER性能进行了比较。我们在此证明,由Cu 2 S的原位电化学氧化产生的CuO对OER表现出非凡的电催化性能,远高于未经这种转化合成的CuO和Cu(OH)2。
更新日期:2019-08-28
中文翻译:

Cu2S原位电化学氧化成CuO纳米线作为一种持久有效的氧析出反应电催化剂
对于部署基于金属-空气电池和可逆燃料电池的大规模储能系统而言,开发具有成本效益的制氧催化剂至关重要。在这个方向上,已经探索了广泛的材料,特别是在更有利的碱性条件下,并且几种金属硫属化物特别表现出优异的性能。然而,在碱性介质中的氧化电势下,硫属化物的热力学稳定性低于相应的氧化物和氢氧化物。尽管这种不稳定性在某些情况下阻止了硫族化物用作制氧催化剂,而在另一些情况下却被忽略,但我们建议使用它来生产高性能的制氧催化剂。我们在这里描述原位化学,结构,2 S转变为CuO纳米线,介导Cu(OH)2的中间形成。我们还测试了它们在碱性介质中在OER下的OER活性和稳定性,并将它们与直接生长在铜网表面的Cu(OH)2和CuO纳米结构的OER性能进行了比较。我们在此证明,由Cu 2 S的原位电化学氧化产生的CuO对OER表现出非凡的电催化性能,远高于未经这种转化合成的CuO和Cu(OH)2。

































 京公网安备 11010802027423号
京公网安备 11010802027423号