当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic Cleavage of Aryl Ether in Modified Lignin to Non-phenolic Aromatics
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-08-14 00:00:00 , DOI: 10.1021/acscatal.9b02719 Hongji Li 1, 2 , Anon Bunrit 1 , Jianmin Lu 1 , Zhuyan Gao 1, 2 , Nengchao Luo 1, 2 , Huifang Liu 1 , Feng Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-08-14 00:00:00 , DOI: 10.1021/acscatal.9b02719 Hongji Li 1, 2 , Anon Bunrit 1 , Jianmin Lu 1 , Zhuyan Gao 1, 2 , Nengchao Luo 1, 2 , Huifang Liu 1 , Feng Wang 1
Affiliation
|
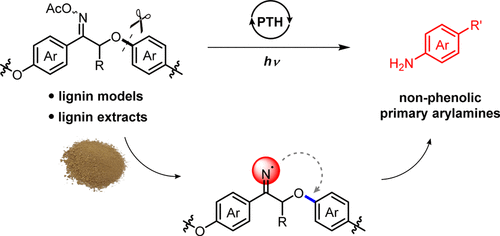
Depolymerization of lignin meets the difficulty in cleaving the robust aryl ether bond. Herein, through installing an internal nucleophile in the β-O-4′ linkage, the selective cleavage of aryl ether was realized by the intramolecular substitution on aryl rings affording non-phenolic arylamine products. In particular, nitrogen-modified lignin models and lignin samples were employed to generate the iminyl radical under photocatalytic reduction, which acted as the internal nucleophile inducing aryl migration from O to the N atom. The following hydrolysis released primary arylamines and α-hydroxy ketones. Mechanism studies including electron spin resonance (ESR), fluorescence quenching experiments, and density functional theory (DFT) calculations proved the aryl migration pathway. This method enables access to non-phenolic arylamine products from lignin conversion.
中文翻译:

改性木质素中芳醚的光催化裂解为非酚芳烃
木质素的解聚遇到了裂解牢固的芳基醚键的困难。在此,通过在β-O-4'键上安装内部亲核试剂,通过在芳环上进行分子内取代而实现芳基醚的选择性裂解,从而得到非酚性芳胺产物。特别地,使用氮改性的木质素模型和木质素样品在光催化还原下产生亚胺基,其充当内部亲核试剂,诱导芳基从O原子迁移到N原子。随后的水解释放出伯芳基胺和α-羟基酮。包括电子自旋共振(ESR),荧光猝灭实验和密度泛函理论(DFT)计算在内的机理研究证明了芳基迁移途径。
更新日期:2019-08-14
中文翻译:

改性木质素中芳醚的光催化裂解为非酚芳烃
木质素的解聚遇到了裂解牢固的芳基醚键的困难。在此,通过在β-O-4'键上安装内部亲核试剂,通过在芳环上进行分子内取代而实现芳基醚的选择性裂解,从而得到非酚性芳胺产物。特别地,使用氮改性的木质素模型和木质素样品在光催化还原下产生亚胺基,其充当内部亲核试剂,诱导芳基从O原子迁移到N原子。随后的水解释放出伯芳基胺和α-羟基酮。包括电子自旋共振(ESR),荧光猝灭实验和密度泛函理论(DFT)计算在内的机理研究证明了芳基迁移途径。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号