当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tumor Microenvironment‐Tailored Weakly Cell‐Interacted Extracellular Delivery Platform Enables Precise Antibody Release and Function
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-08-15 , DOI: 10.1002/adfm.201903296 Sidi Li 1 , Luyang Chen 1 , Kai Huang 2 , Ning Chen 1 , Qi Zhan 1 , Kaikai Yi 2 , Hongzhao Qi 1, 3 , Chaoyong Liu 1, 2, 4 , Yanli Tan 5 , Xin Hou 1 , Yunfeng Lu 4 , Jin Zhao 1 , Xubo Yuan 1 , Chunsheng Kang 2
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-08-15 , DOI: 10.1002/adfm.201903296 Sidi Li 1 , Luyang Chen 1 , Kai Huang 2 , Ning Chen 1 , Qi Zhan 1 , Kaikai Yi 2 , Hongzhao Qi 1, 3 , Chaoyong Liu 1, 2, 4 , Yanli Tan 5 , Xin Hou 1 , Yunfeng Lu 4 , Jin Zhao 1 , Xubo Yuan 1 , Chunsheng Kang 2
Affiliation
|
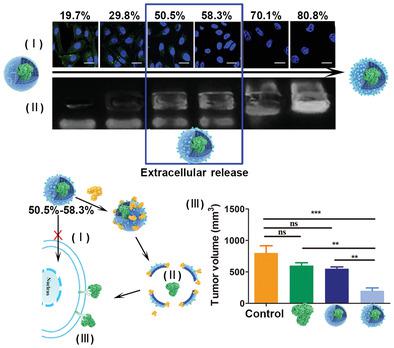
Precise delivery of extracellularly functional protein drugs is limited by the drawback in that the protective carrier often causes undesirable cellular uptake of these therapeutic agents. Here, the design of a weakly cell‐interacted, nanosized, environment‐responsive vehicle (WINNER) with rational phosphorylcholine (PC) surface filling ratios capable of precise extracellular delivery of therapeutic agents for enhanced tumor suppression is reported. Highly hydrophilic zwitterionic PC and enzyme‐responsive peptides are engineered into the functional shell of WINNER which reasonably covers the inner protein. It is demonstrated that rationally controlled PC surface filling ratios (50.5–58.3%) are necessary for weakening interactions between the cell and WINNER whilst providing enough sites on WINNER for enzyme recognition. Consequently, WINNER (50.5–58.3%) can protect inner cargos from cellular uptake and undergo enzymatic degradation, resulting in precise extracellular release of inner protein, such as therapeutic monoclonal antibody (mAb). After intravenous administration, therapeutic mAb nimotuzumab‐loaded WINNER (51.2%) shows highest in vivo antitumor activity compared with free nimotuzumab or nimotuzumab‐loaded PC‐free nanocarrier in a lung adenocarcinoma xenograft tumor animal model. This work presents a simple and flexible approach to design precise extracellular delivery platform which can uncage the therapeutic power of extracellular targeting therapeutic agents.
中文翻译:

肿瘤微环境修饰的弱细胞相互作用的细胞外递送平台可实现精确的抗体释放和功能
细胞外功能蛋白药物的精确递送受到以下缺点的限制:保护性载体通常引起这些治疗剂的不希望的细胞摄取。在此,报道了一种设计成具有合理的磷酸胆碱(PC)表面填充率,能够精确地将细胞外递送治疗剂以增强肿瘤抑制作用的弱细胞相互作用,纳米级,环境响应性载体(WINNER)的设计。高度亲水的两性离子PC和酶促反应肽被工程化到WINNER的功能外壳中,该外壳合理地覆盖了内部蛋白质。结果表明,必须合理控制PC表面填充率(50.5-58.3%),才能减弱细胞与WINNER之间的相互作用,同时在WINNER上提供足够的酶识别位点。因此,WINNER(50。5-58.3%)可以保护内部货物免受细胞摄取并发生酶促降解,从而导致内部蛋白质(例如治疗性单克隆抗体(mAb))的精确胞外释放。静脉内给药后,在肺腺癌异种移植肿瘤动物模型中,载有mAb尼妥珠单抗的WINNER(51.2%)与游离尼妥珠单抗或不含尼莫妥珠单抗的不含PC纳米载体相比具有最高的体内抗肿瘤活性。这项工作提出了一种简单而灵活的方法来设计精确的细胞外递送平台,该平台可以消除细胞外靶向治疗剂的治疗能力。静脉内给药后,在肺腺癌异种移植肿瘤动物模型中,载有mAb尼妥珠单抗的WINNER(51.2%)与游离尼妥珠单抗或不含尼莫妥珠单抗的不含PC纳米载体相比具有最高的体内抗肿瘤活性。这项工作提出了一种简单而灵活的方法来设计精确的细胞外递送平台,该平台可以消除细胞外靶向治疗剂的治疗能力。静脉内给药后,在肺腺癌异种移植肿瘤动物模型中,载有mAb尼妥珠单抗的WINNER(51.2%)与游离尼妥珠单抗或不含尼莫妥珠单抗的不含PC纳米载体相比具有最高的体内抗肿瘤活性。这项工作提出了一种简单而灵活的方法来设计精确的细胞外递送平台,该平台可以消除细胞外靶向治疗剂的治疗能力。
更新日期:2019-10-23
中文翻译:

肿瘤微环境修饰的弱细胞相互作用的细胞外递送平台可实现精确的抗体释放和功能
细胞外功能蛋白药物的精确递送受到以下缺点的限制:保护性载体通常引起这些治疗剂的不希望的细胞摄取。在此,报道了一种设计成具有合理的磷酸胆碱(PC)表面填充率,能够精确地将细胞外递送治疗剂以增强肿瘤抑制作用的弱细胞相互作用,纳米级,环境响应性载体(WINNER)的设计。高度亲水的两性离子PC和酶促反应肽被工程化到WINNER的功能外壳中,该外壳合理地覆盖了内部蛋白质。结果表明,必须合理控制PC表面填充率(50.5-58.3%),才能减弱细胞与WINNER之间的相互作用,同时在WINNER上提供足够的酶识别位点。因此,WINNER(50。5-58.3%)可以保护内部货物免受细胞摄取并发生酶促降解,从而导致内部蛋白质(例如治疗性单克隆抗体(mAb))的精确胞外释放。静脉内给药后,在肺腺癌异种移植肿瘤动物模型中,载有mAb尼妥珠单抗的WINNER(51.2%)与游离尼妥珠单抗或不含尼莫妥珠单抗的不含PC纳米载体相比具有最高的体内抗肿瘤活性。这项工作提出了一种简单而灵活的方法来设计精确的细胞外递送平台,该平台可以消除细胞外靶向治疗剂的治疗能力。静脉内给药后,在肺腺癌异种移植肿瘤动物模型中,载有mAb尼妥珠单抗的WINNER(51.2%)与游离尼妥珠单抗或不含尼莫妥珠单抗的不含PC纳米载体相比具有最高的体内抗肿瘤活性。这项工作提出了一种简单而灵活的方法来设计精确的细胞外递送平台,该平台可以消除细胞外靶向治疗剂的治疗能力。静脉内给药后,在肺腺癌异种移植肿瘤动物模型中,载有mAb尼妥珠单抗的WINNER(51.2%)与游离尼妥珠单抗或不含尼莫妥珠单抗的不含PC纳米载体相比具有最高的体内抗肿瘤活性。这项工作提出了一种简单而灵活的方法来设计精确的细胞外递送平台,该平台可以消除细胞外靶向治疗剂的治疗能力。































 京公网安备 11010802027423号
京公网安备 11010802027423号