当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Concise Stereoselective Synthesis of (R)‐2‐Benzylmorpholine and ML398 from (R)‐(−)‐2‐Phenylglycinol
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2019-08-13 , DOI: 10.1002/jhet.3657 Saúl Torres 1 , Manuel Velasco 1 , José Ángel Gallegos‐Rojas 2 , Sylvain Bernès 3 , María L. Orea 1 , Joel L. Terán 1 , Gabriela Huelgas 4 , Víctor Gómez‐Calvario 1 , Jorge R. Juárez 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2019-08-13 , DOI: 10.1002/jhet.3657 Saúl Torres 1 , Manuel Velasco 1 , José Ángel Gallegos‐Rojas 2 , Sylvain Bernès 3 , María L. Orea 1 , Joel L. Terán 1 , Gabriela Huelgas 4 , Víctor Gómez‐Calvario 1 , Jorge R. Juárez 1
Affiliation
|
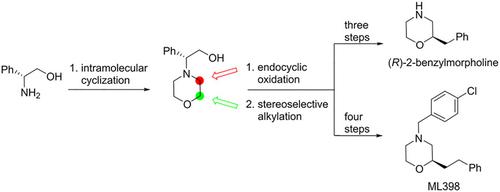
We describe here an efficient stereoselective method for the preparation of (R)‐2‐benzylmorpholine and ML398. The present method features a high diastereocontrol using an endocyclic oxidation of phenylglycinol‐derived morpholine and a stereoselective alkylation of chiral non‐racemic morpholin‐3‐one as key steps.
中文翻译:

(R)-2-苄基吗啉和ML398的立体立体选择性合成(R)-(-)-2-苯基甘氨醇
我们在这里描述了一种有效的立体选择性方法,用于制备(R)-2-苄基吗啉和ML398。本方法具有高非对映控制性,其中关键步骤是使用苯甘醇衍生的吗啉的内环氧化和手性非外消旋吗啉-3-酮的立体选择性烷基化。
更新日期:2019-08-13
中文翻译:

(R)-2-苄基吗啉和ML398的立体立体选择性合成(R)-(-)-2-苯基甘氨醇
我们在这里描述了一种有效的立体选择性方法,用于制备(R)-2-苄基吗啉和ML398。本方法具有高非对映控制性,其中关键步骤是使用苯甘醇衍生的吗啉的内环氧化和手性非外消旋吗啉-3-酮的立体选择性烷基化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号