当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Palodesangren B Trimethyl Ether and D Dimethyl Ether via a Late-Stage Formation of 2H-Pyran-2-one of the Tetrahydrobenzo[c]pyranochromenone Core.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-08-27 , DOI: 10.1021/acs.joc.9b01596 Kassrin Tangdenpaisal 1 , Poramate Songthammawat 2 , Kornkamon Akkarasereenon 2 , Kanokpish Chuayboonsong 2 , Somsak Ruchirawat 1, 2, 3 , Poonsakdi Ploypradith 1, 2, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-08-27 , DOI: 10.1021/acs.joc.9b01596 Kassrin Tangdenpaisal 1 , Poramate Songthammawat 2 , Kornkamon Akkarasereenon 2 , Kanokpish Chuayboonsong 2 , Somsak Ruchirawat 1, 2, 3 , Poonsakdi Ploypradith 1, 2, 3
Affiliation
|
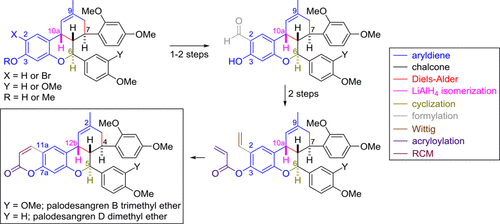
In four steps from the tricyclic core, palodesangren B trimethyl ether and palodesangren D dimethyl ether could be synthesized in 29 and 18% overall yields, respectively. A reaction sequence comprising the regioselective MgCl2-mediated Casnati-Skattebøl ortho-formylation of phenol, Wittig methylenation, acryloylation, and Ru(II)-catalyzed ring-closing metathesis (RCM) led to the formation of the final 2H-pyran-2-one ring of the desired tetracyclic core.
中文翻译:

通过四氢苯并[c]吡喃并色酮核的2H-吡喃-2-酮的后期形成,完全合成帕洛丹桑BB三甲基醚和D二甲基醚。
在三环核的四个步骤中,可以分别以29%和18%的总产率合成palodesangren B三甲基醚和palodesangren D二甲基醚。包含区域选择性MgCl2介导的Casnati-Skattebøl苯酚的甲酰化,Wittig甲基化,丙烯酰化和Ru(II)催化的闭环复分解(RCM)的反应序列导致最终2H-pyran-2-的形成所需四环核的一个环。
更新日期:2019-08-27
中文翻译:

通过四氢苯并[c]吡喃并色酮核的2H-吡喃-2-酮的后期形成,完全合成帕洛丹桑BB三甲基醚和D二甲基醚。
在三环核的四个步骤中,可以分别以29%和18%的总产率合成palodesangren B三甲基醚和palodesangren D二甲基醚。包含区域选择性MgCl2介导的Casnati-Skattebøl苯酚的甲酰化,Wittig甲基化,丙烯酰化和Ru(II)催化的闭环复分解(RCM)的反应序列导致最终2H-pyran-2-的形成所需四环核的一个环。































 京公网安备 11010802027423号
京公网安备 11010802027423号