Ecotoxicology and Environmental Safety ( IF 6.2 ) Pub Date : 2019-08-13 , DOI: 10.1016/j.ecoenv.2019.109551 Wen Liu , Guochun Lv , Xiaomin Sun , Lin He , Chenxi Zhang , Zhiqiang Li
|
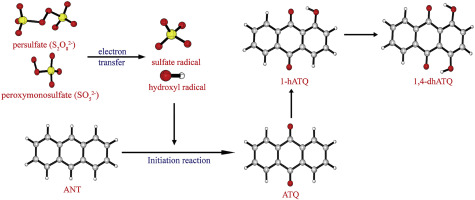
Sulfate radical (SO4−) and hydroxyl radical (
OH) generated from persulfate or peroxymonosulfate in AOPs have been widely used in contaminant degradation. Anthracene (ANT) can be decomposed by SO4
− and
OH. The processes of ANT decomposition were investigated using theoretical calculations in this paper. The initiation reactions of ANT, anthrone, anthraquinone (ATQ) and 1-hydroxylanthraquinone (1-hATQ) by two radicals are studied. The highest free energy barriers of initiation reactions are 22.30 kcal mol−1 in ATQ + SO4
− reaction and 6.84 kcal mol−1 in ATQ +
OH reaction. Comparing the rate constants of initiation reaction through the two radicals at 273–373 K, it can be concluded that SO4
− and
OH both play important roles on the initiation of ANT and anthrone at lower pH. For ATQ and 1-hATQ,
OH is more important than SO4
− in the initiation process, which indicates that the indirect influence of SO4
− are more significant in the degradation processes of ATQ and 1-hATQ. This study provides theoretical confirmations for the mechanisms of reactions of ANT with SO4
− and
OH, and evaluates the importance of SO4
− and
OH according to the reaction rates. The work can give more insight into the degradation of PAHs by radicals.
中文翻译:

蒽与水溶液中硫酸根和羟基的反应的理论研究
硫酸根(SO 4 - )和羟基自由基(
从过硫酸盐或过氧单产生在高级氧化已被广泛用于在污染物降解OH)。蒽(ANT)可以通过SO分解4
-和
OH。本文采用理论计算方法研究了ANT的分解过程。研究了两个自由基对ANT,蒽酮,蒽醌(ATQ)和1-羟基镧醌(1-hATQ)的引发反应。开始反应的最高自由能屏障是22.30千卡摩尔-1在ATQ + SO 4
-反应和6.84千卡摩尔-1在ATQ +
OH反应。通过比较在273-373 k处的两个基团引发反应的速率常数,可以得出结论,SO 4
-和
OH对ANT的启动和蒽酮在较低的pH值都起着重要的作用。对于ATQ和1- hATQ,
OH比SO更重要4
-在启动过程中,这表明的SO间接影响4
-是在ATQ和1- hATQ的降解过程更显著。本研究为ANT的反应的机理理论确认与SO 4
-和
OH,并且评估SO的重要性4
-和
OH根据反应速率而定。这项工作可以使人们更深入地研究自由基对PAHs的降解作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号