当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aminoalkylation of [1.1.1]Propellane Enables Direct Access to High-Value 3-Alkylbicyclo[1.1.1]pentan-1-amines.
Organic Letters ( IF 4.9 ) Pub Date : 2019-08-13 00:00:00 , DOI: 10.1021/acs.orglett.9b02426 Jonathan M E Hughes 1 , David A Scarlata 1 , Austin C-Y Chen 2 , Jason D Burch 3 , James L Gleason 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-08-13 00:00:00 , DOI: 10.1021/acs.orglett.9b02426 Jonathan M E Hughes 1 , David A Scarlata 1 , Austin C-Y Chen 2 , Jason D Burch 3 , James L Gleason 1
Affiliation
|
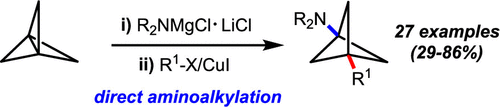
Bicyclo[1.1.1]pentanes are effective bioisoteres for aromatic rings, tert-butyl groups, and alkynes. Here we report the first method to synthesize 3-alkylbicyclo[1.1.1]pentan-1-amines directly from [1.1.1]propellane via sequential addition of magnesium amides and alkyl electrophiles. The mild reaction conditions tolerate a variety of important functional groups and enable efficient incorporation of several pharmaceutically relevant amines onto the bicyclo[1.1.1]pentane scaffold. This method's utility is highlighted by its ability to significantly streamline the syntheses of several important bicyclo[1.1.1]pentan-1-amine building blocks.
中文翻译:

[1.1.1]环戊烷的氨基烷基化可直接获得高价值的3-烷基双环[1.1.1]戊烷-1-胺。
双环[1.1.1]戊烷是芳香环,叔丁基和炔烃的有效生物异构体。在这里,我们报告的第一种方法是通过依次添加酰胺化镁和烷基亲电试剂,直接从[1.1.1]丙炔直接合成3-烷基双环[1.1.1]戊丹-1-胺。温和的反应条件可以耐受各种重要的官能团,并能够将几种药学上相关的胺有效地结合到双环[1.1.1]戊烷骨架上。该方法的实用性因其能够显着简化几种重要的双环[1.1.1]戊丹-1-胺结构单元的合成能力而得到强调。
更新日期:2019-08-13
中文翻译:

[1.1.1]环戊烷的氨基烷基化可直接获得高价值的3-烷基双环[1.1.1]戊烷-1-胺。
双环[1.1.1]戊烷是芳香环,叔丁基和炔烃的有效生物异构体。在这里,我们报告的第一种方法是通过依次添加酰胺化镁和烷基亲电试剂,直接从[1.1.1]丙炔直接合成3-烷基双环[1.1.1]戊丹-1-胺。温和的反应条件可以耐受各种重要的官能团,并能够将几种药学上相关的胺有效地结合到双环[1.1.1]戊烷骨架上。该方法的实用性因其能够显着简化几种重要的双环[1.1.1]戊丹-1-胺结构单元的合成能力而得到强调。































 京公网安备 11010802027423号
京公网安备 11010802027423号