当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Bioactivity of the Alanyl Phosphonamidate Stereoisomers Derived from a Butyrophilin Ligand.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-08-13 , DOI: 10.1021/acsmedchemlett.9b00153 Nicholas A Lentini 1 , Chia-Hung Christine Hsiao 2 , George B Crull 1 , Andrew J Wiemer 2, 3 , David F Wiemer 1, 4
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-08-13 , DOI: 10.1021/acsmedchemlett.9b00153 Nicholas A Lentini 1 , Chia-Hung Christine Hsiao 2 , George B Crull 1 , Andrew J Wiemer 2, 3 , David F Wiemer 1, 4
Affiliation
|
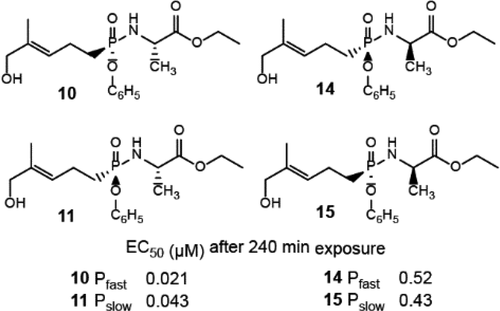
Aryloxy phosphonamidate derivatives of a butyrophilin 3A1 ligand are stimulants of Vγ9 Vδ2 T cells. However, when bonded to an aryl ester and an amine, the phosphorus is stereogenic, and past compounds were studied as racemates. To determine the impact of stereochemistry on the activity, we now have prepared phosphonate derivatives of l- and d-alanine ethyl ester, separated the diastereomers, and evaluated their biological activity as single stereoisomers. The results demonstrate that phosphonamidates substituted with l-alanine stimulate Vγ9 Vδ2 T cells at lower concentrations than the racemic glycine counterpart, while those derived from d-alanine require higher concentrations. All four diastereomers are more active than charged phosphoantigens such as HMBPP. Surprisingly, only a 2-fold difference was observed between the l-alanine phosphorus isomers, with the RP isomer more potent. This suggests that the small phosphoantigen scaffold reduces but does not eliminate dependence upon phosphorus stereochemistry for cellular activity.
中文翻译:

衍生自Butyrophilin配体的戊酰胺基氨基磺酸膦酸酯立体异构体的合成和生物活性。
嗜丁菌素3A1配体的芳氧基膦酸酯酰胺衍生物是Vγ9Vδ2T细胞的刺激物。然而,当与芳基酯和胺键合时,磷是立体异构的,过去的化合物已被研究为外消旋体。为了确定立体化学对活性的影响,我们现在制备了1-和d-丙氨酸乙酯的膦酸酯衍生物,分离了非对映异构体,并评估了它们作为单一立体异构体的生物学活性。结果表明,被1-丙氨酸取代的膦酰胺能以比消旋甘氨酸对应物更低的浓度刺激Vγ9Vδ2T细胞,而那些衍生自d-丙氨酸需要更高的浓度。所有四种非对映异构体均比带电荷的磷酸抗原(如HMBPP)更具活性。令人惊讶地,在1-丙氨酸磷异构体之间仅观察到2倍的差异,而R P异构体更有效。这表明小的磷酸抗原支架减少但不能消除对磷立体化学对细胞活性的依赖性。
更新日期:2019-08-13
中文翻译:

衍生自Butyrophilin配体的戊酰胺基氨基磺酸膦酸酯立体异构体的合成和生物活性。
嗜丁菌素3A1配体的芳氧基膦酸酯酰胺衍生物是Vγ9Vδ2T细胞的刺激物。然而,当与芳基酯和胺键合时,磷是立体异构的,过去的化合物已被研究为外消旋体。为了确定立体化学对活性的影响,我们现在制备了1-和d-丙氨酸乙酯的膦酸酯衍生物,分离了非对映异构体,并评估了它们作为单一立体异构体的生物学活性。结果表明,被1-丙氨酸取代的膦酰胺能以比消旋甘氨酸对应物更低的浓度刺激Vγ9Vδ2T细胞,而那些衍生自d-丙氨酸需要更高的浓度。所有四种非对映异构体均比带电荷的磷酸抗原(如HMBPP)更具活性。令人惊讶地,在1-丙氨酸磷异构体之间仅观察到2倍的差异,而R P异构体更有效。这表明小的磷酸抗原支架减少但不能消除对磷立体化学对细胞活性的依赖性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号