Cell Death Discovery ( IF 6.1 ) Pub Date : 2019-08-13 , DOI: 10.1038/s41420-019-0209-z
Hongyan Wei , Meixian Yin , Yuanzheng Lu , Yan Yang , Bo Li , Xiao-Xing Liao , Gang Dai , Xiaoli Jing , Yan Xiong , Chunlin Hu
|
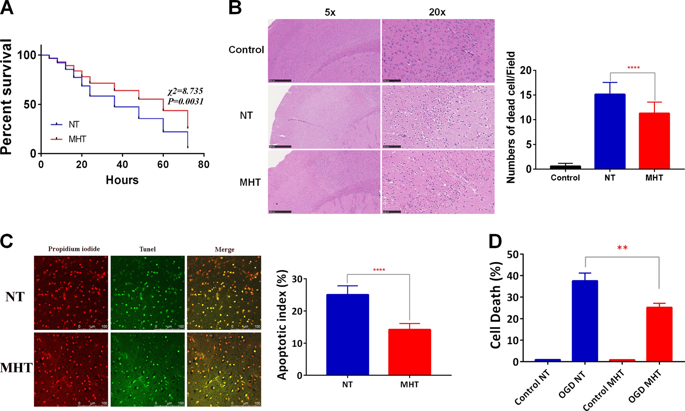
Mild hypothermia treatment (MHT) improves the neurological function of cardiac arrest (CA) patients, but the exact mechanisms of recovery remain unclear. Herein, we generated a CA and cardiopulmonary resuscitation (CPR) mouse model to elucidate such function. Naïve mice were randomly divided into two groups, a normothemia (NT) group, in which animals had normal body temperature, and a MHT group, in which animals had a body temperature of 33 °C (range: 32–34 °C), after the return of spontaneous circulation (ROSC), followed by CA/CPR. MHT significantly improved the survival rate of CA/CPR mice compared with NT. Mechanistically, MHT increased the expression of Silent Information Regulator 1 (Sirt1) and decreased P53 phosphorylation (p-P53) in the cortex of CA/CPR mice, which coincided with the elevated autophagic flux. However, Sirt1 deletion compromised the neuroprotection offered by MHT, indicating that Sirt1 plays an important role. Consistent with the observations obtained from in vivo work, our in vitro study utilizing cultured neurons subjected to oxygen/glucose deprivation and reperfusion (OGD/R) also indicated that Sirt1 knockdown increased OGD/R-induced neuron necrosis and apoptosis, which was accompanied by decreased autophagic flux and increased p-P53. However, the depletion of P53 did not suppress neuron death, suggesting that P53 was not critically involved in MHT-induced neuroprotection. In contrast, the application of autophagic inhibitor 3-methyladenine attenuated MHT-improved neuron survival after OGD/R, further demonstrating that increased autophagic flux significantly contributes to MHT-linked neuroprotection of CA/CRP mice. Our findings indicate that MHT improves neurological outcome of mice after CA/CPR through Sirt1-mediated activation of autophagic flux.
中文翻译:

轻微的体温过低可通过沉默信息调节剂1激活的自体吞噬改善心肺复苏后小鼠的神经系统预后
温和的低温治疗(MHT)可以改善心脏骤停(CA)患者的神经功能,但恢复的确切机制仍不清楚。在这里,我们生成了一个CA和心肺复苏(CPR)小鼠模型来阐明这种功能。幼稚的小鼠随机分为两组,正常动物的体温正常(NT)组和体温为33°C(范围:32–34°C)的MHT组,自发循环(ROSC)恢复后,接着是CA / CPR。与NT相比,MHT显着提高了CA / CPR小鼠的存活率。从机制上讲,MHT增加了CA / CPR小鼠皮层中Silent Information Regulator 1(Sirt1)的表达并降低了P53磷酸化(p-P53),这与自噬通量升高相吻合。然而,Sirt1缺失损害了MHT提供的神经保护作用,表明Sirt1发挥重要作用。与从体内获得的观察结果一致,我们利用经过氧/葡萄糖剥夺和再灌注(OGD / R)的培养神经元进行的体外研究还表明,Sirt1敲低增加了OGD / R诱导的神经元坏死和细胞凋亡,这伴随着自噬通量降低,p-P53升高。但是,P53的耗竭并不能抑制神经元的死亡,这表明P53并不参与MHT诱导的神经保护作用。相比之下,自噬抑制剂3-甲基腺嘌呤的应用减弱了OGD / R后MHT改善的神经元存活,进一步证明自噬通量的增加显着促进了CA / CRP小鼠的MHT连锁神经保护。

































 京公网安备 11010802027423号
京公网安备 11010802027423号