Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-08-12 , DOI: 10.1016/j.tetlet.2019.151040 Mulla Althafh Hussain , Faiz Ahmed Khan
|
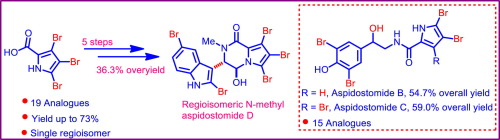
A full account of the total synthesis of aspidostomide B, C, their analogues and our synthetic efforts towards the synthesis of aspidostomide D, which led to the synthesis of regioisomeric N-methyl aspidostomide D, its analogues via epoxide opening strategy is presented. The synthesis of regioisomeric N-methyl aspidostomide D involves an efficient, five-step sequence, with 36.3% overall yield, starting from 3,4,5-tribromo-1H-pyrrole-2-carboxylic acid. The key features of this protocol are intramolecular cyclization, dehydration, oxidation, and a Lewis acid-mediated regioselective epoxide ring opening by C-3 position of 2,5-dibromo-1H-indole to furnish the title compounds.
中文翻译:

(±)毒杀霉菌素B,C,区域异构体N-甲基毒杀霉菌素D及其衍生物的全合成
全面介绍了杀虫威B,C,其类似物的总合成以及我们为合成杀虫威D而进行的合成努力,该合成导致了通过环氧化物开放策略合成区域异构体N-甲基杀虫威D。从3,4,5-三溴-1H-吡咯-2-羧酸开始,区域异构体N-甲基蛇毒苷D的合成涉及有效的五步序列,总收率达36.3%。该方案的关键特征是分子内环化,脱水,氧化和路易斯酸介导的区域选择性环氧化物环的2,5-二溴-1H-吲哚的C-3位置开环,以提供标题化合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号