当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploiting Hydrazones To Improve the Efficiency of 6π-Electrocyclization Reactions of 1-Azatrienes.
Organic Letters ( IF 4.9 ) Pub Date : 2019-08-12 00:00:00 , DOI: 10.1021/acs.orglett.9b02455 Matthew P Ball-Jones 1 , Jasper Tyler 1 , Helena Mora-Radó 1, 2 , Werngard Czechtizky 2 , María Méndez 2 , Joseph P A Harrity 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-08-12 00:00:00 , DOI: 10.1021/acs.orglett.9b02455 Matthew P Ball-Jones 1 , Jasper Tyler 1 , Helena Mora-Radó 1, 2 , Werngard Czechtizky 2 , María Méndez 2 , Joseph P A Harrity 1
Affiliation
|
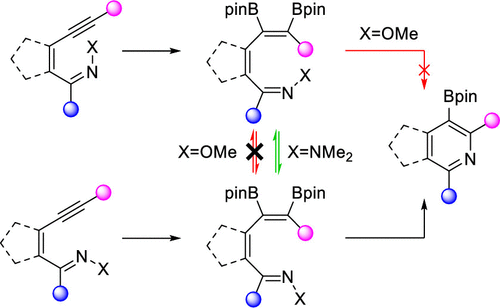
The greater geometric lability of hydrazones compared to that of oxime ethers is used as a basis to overcome the reluctance of Z-oxime ether azatrienes to undergo electrocyclization toward the synthesis of borylated (heteroaromatic) pyridines and ring-fused analogues. Such hydrazones now allow access to previously inaccessible tri- and tetrasubstituted 3-borylpyridines in high yields.
中文翻译:

开发Hy提高1-氮杂三烯的6π-电环化反应的效率。
与肟醚相比,具有更大的几何不稳定性,被用作克服Z-肟醚氮杂三烯不愿意进行电环化以合成硼酸化(杂芳族)吡啶和环稠合类似物的基础。这种现在可以以高收率获得以前难以获得的三和四取代的3-硼基吡啶。
更新日期:2019-08-12
中文翻译:

开发Hy提高1-氮杂三烯的6π-电环化反应的效率。
与肟醚相比,具有更大的几何不稳定性,被用作克服Z-肟醚氮杂三烯不愿意进行电环化以合成硼酸化(杂芳族)吡啶和环稠合类似物的基础。这种现在可以以高收率获得以前难以获得的三和四取代的3-硼基吡啶。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号