当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fe2+/Fe3+ Ions Chelated with Ultrasmall Polydopamine Nanoparticles Induce Ferroptosis for Cancer Therapy
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2019-08-12 00:00:00 , DOI: 10.1021/acsbiomaterials.9b00461 Lu Chen 1 , Zhenjie Lin 1 , Lizhu Liu 1 , Xiuming Zhang 1 , Wei Shi 1 , Dongtao Ge 1 , Yanan Sun 1
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2019-08-12 00:00:00 , DOI: 10.1021/acsbiomaterials.9b00461 Lu Chen 1 , Zhenjie Lin 1 , Lizhu Liu 1 , Xiuming Zhang 1 , Wei Shi 1 , Dongtao Ge 1 , Yanan Sun 1
Affiliation
|
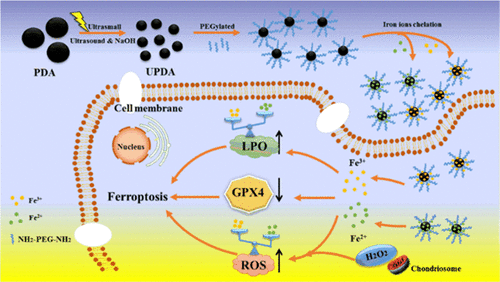
Ferroptosis, a promising mechanism of killing cancer cells, has become a research hotspot in cancer therapy. Besides, advantages of polymeric nanomaterials in improving anticancer efficacy and reducing side effect are widely accepted. In this work, based on the property of polypodamine to chelate metal ions, ultrasmall poly(ethylene glycol)-modified polydopamine nanoparticles, (UPDA-PEG)@Fe2+/3+ nanoparticles, a novel ferroptosis agent, was rationally designed by chelating iron ions on ultrasmall polydopamine nanoparticles modified by PEG. This treatment led to a bigger specific surface area, which could support more reactive sites to chelate large number of iron ions, which is beneficial for exploring the detailed mechanism of ferroptosis-induced tumor cell death by iron ions. Also, the pH-dependent release of iron ions can reach approximately 70% at pH 5.0, providing the advantage of application in tumor microenvironment. The in vitro tests showed that the as-prepared NPs exhibit an effective anticancer effect on tumor cells including 4T1 and U87MG cells, yet ferric ions show a stronger ability of killing cancer cells than ferrous ions. Differences between ferrous ions and ferric ions in the ferroptosis pathway were monitored by the change of marker, including reactive oxygen species (ROS), glutathione peroxidase 4, and lipid peroxide (LPO), as well as the promoter and inhibitor of ferroptosis pathway. [email protected]2+ nanoparticles induce ferroptosis that depends more on ROS; however, a more LPO-dependent ferroptosis is induced by [email protected]3+ nanoparticles. Additionally, the in vivo studies using tumor-bearing Balb/c mice demonstrated that the as-prepared NPs could significantly inhibit tumor progression. [email protected]2+/3+ nanoparticles reported herein represent the nanoparticles related to iron ions for chemotherapy against cancer through the ferroptosis pathway.
中文翻译:

Fe 2+ / Fe 3+离子与超小聚多巴胺纳米粒子螯合可诱导肥大病的治疗
Ferroptosis是杀死癌细胞的一种有前途的机制,已成为癌症治疗的研究热点。此外,聚合物纳米材料在提高抗癌功效和减少副作用方面的优点被广泛接受。在这项工作中,基于聚多巴胺螯合金属离子的特性,制备了超小型聚乙二醇修饰的聚多巴胺纳米粒子(UPDA-PEG)@ Fe 2 + / 3 +通过在PEG修饰的超小型聚多巴胺纳米颗粒上螯合铁离子,合理设计了一种新型的肥大症治疗剂纳米颗粒。该处理导致更大的比表面积,其可以支持更多的反应位点以螯合大量铁离子,这有利于探索铁离子引起的肥大病引起的肿瘤细胞死亡的详细机理。而且,pH依赖的铁离子释放在pH 5.0时可达到约70%,提供了在肿瘤微环境中应用的优势。体外试验表明,所制备的纳米粒对包括4T1和U87MG细胞在内的肿瘤细胞具有有效的抗癌作用,而三价铁离子比二价离子具有更强的杀死癌细胞的能力。通过标志物的变化,监测了铁素体形成途径中亚铁离子和铁离子之间的差异,包括活性氧(ROS),谷胱甘肽过氧化物酶4和脂质过氧化物(LPO)以及铁素体形成途径的启动子和抑制剂。[电子邮件保护]2+纳米颗粒会引起铁氧体肥大,而铁氧体铁更依赖于ROS。但是,[电子邮件保护的] 3+纳米颗粒诱导了更多的LPO依赖型肥大症。此外,使用荷瘤Balb / c小鼠的体内研究表明,制备的NPs可以显着抑制肿瘤的进展。本文报道的[电子邮件保护的] 2 + / 3 +纳米颗粒代表与铁离子有关的纳米颗粒,用于通过铁质增生途径进行化学疗法抗癌。
更新日期:2019-08-12
中文翻译:

Fe 2+ / Fe 3+离子与超小聚多巴胺纳米粒子螯合可诱导肥大病的治疗
Ferroptosis是杀死癌细胞的一种有前途的机制,已成为癌症治疗的研究热点。此外,聚合物纳米材料在提高抗癌功效和减少副作用方面的优点被广泛接受。在这项工作中,基于聚多巴胺螯合金属离子的特性,制备了超小型聚乙二醇修饰的聚多巴胺纳米粒子(UPDA-PEG)@ Fe 2 + / 3 +通过在PEG修饰的超小型聚多巴胺纳米颗粒上螯合铁离子,合理设计了一种新型的肥大症治疗剂纳米颗粒。该处理导致更大的比表面积,其可以支持更多的反应位点以螯合大量铁离子,这有利于探索铁离子引起的肥大病引起的肿瘤细胞死亡的详细机理。而且,pH依赖的铁离子释放在pH 5.0时可达到约70%,提供了在肿瘤微环境中应用的优势。体外试验表明,所制备的纳米粒对包括4T1和U87MG细胞在内的肿瘤细胞具有有效的抗癌作用,而三价铁离子比二价离子具有更强的杀死癌细胞的能力。通过标志物的变化,监测了铁素体形成途径中亚铁离子和铁离子之间的差异,包括活性氧(ROS),谷胱甘肽过氧化物酶4和脂质过氧化物(LPO)以及铁素体形成途径的启动子和抑制剂。[电子邮件保护]2+纳米颗粒会引起铁氧体肥大,而铁氧体铁更依赖于ROS。但是,[电子邮件保护的] 3+纳米颗粒诱导了更多的LPO依赖型肥大症。此外,使用荷瘤Balb / c小鼠的体内研究表明,制备的NPs可以显着抑制肿瘤的进展。本文报道的[电子邮件保护的] 2 + / 3 +纳米颗粒代表与铁离子有关的纳米颗粒,用于通过铁质增生途径进行化学疗法抗癌。

































 京公网安备 11010802027423号
京公网安备 11010802027423号