当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
O-GlcNAc transferase recognizes protein substrates using an asparagine ladder in the TPR superhelix
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-02-27 , DOI: 10.1021/jacs.7b13546 Zebulon G Levine 1 , Chenguang Fan 1 , Michael S Melicher 1 , Marina Orman 1 , Tania Benjamin 1 , Suzanne Walker 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-02-27 , DOI: 10.1021/jacs.7b13546 Zebulon G Levine 1 , Chenguang Fan 1 , Michael S Melicher 1 , Marina Orman 1 , Tania Benjamin 1 , Suzanne Walker 1
Affiliation
|
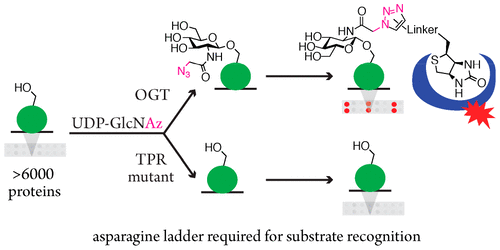
The essential mammalian enzyme O-GlcNAc Transferase (OGT) is uniquely responsible for transferring N-acetylglucosamine to over a thousand nuclear and cytoplasmic proteins, yet there is no known consensus sequence and it remains unclear how OGT recognizes its substrates. To address this question, we developed a protein microarray assay that chemoenzymatically labels de novo sites of glycosylation with biotin, allowing us to simultaneously assess OGT activity across >6000 human proteins. With this assay we examined the contribution to substrate selection of a conserved asparagine ladder within the lumen of OGT's superhelical tetratricopeptide repeat (TPR) domain. When five asparagines were mutated, OGT retained significant activity against short peptides, but showed limited limited glycosylation of protein substrates on the microarray. O-GlcNAcylation of protein substrates in cell extracts was also greatly attenuated. We conclude that OGT recognizes the majority of its substrates by binding them to the asparagine ladder in the TPR lumen proximal to the catalytic domain.
中文翻译:

O-GlcNAc 转移酶使用 TPR 超螺旋中的天冬酰胺梯识别蛋白质底物
哺乳动物必需酶 O-GlcNAc 转移酶 (OGT) 独特地负责将 N-乙酰氨基葡萄糖转移至一千多种核蛋白和细胞质蛋白,但尚无已知的共有序列,并且目前尚不清楚 OGT 如何识别其底物。为了解决这个问题,我们开发了一种蛋白质微阵列测定法,可以用生物素以化学酶方式从头标记糖基化位点,使我们能够同时评估超过 6000 种人类蛋白质的 OGT 活性。通过该测定,我们检查了 OGT 超螺旋四肽重复 (TPR) 结构域内的保守天冬酰胺梯对底物选择的贡献。当五个天冬酰胺发生突变时,OGT 保留了针对短肽的显着活性,但在微阵列上显示出蛋白质底物的有限糖基化。细胞提取物中蛋白质底物的O-GlcNAc酰化也大大减弱。我们得出的结论是,OGT 通过将其结合到靠近催化结构域的 TPR 腔中的天冬酰胺梯来识别其大部分底物。
更新日期:2018-02-27
中文翻译:

O-GlcNAc 转移酶使用 TPR 超螺旋中的天冬酰胺梯识别蛋白质底物
哺乳动物必需酶 O-GlcNAc 转移酶 (OGT) 独特地负责将 N-乙酰氨基葡萄糖转移至一千多种核蛋白和细胞质蛋白,但尚无已知的共有序列,并且目前尚不清楚 OGT 如何识别其底物。为了解决这个问题,我们开发了一种蛋白质微阵列测定法,可以用生物素以化学酶方式从头标记糖基化位点,使我们能够同时评估超过 6000 种人类蛋白质的 OGT 活性。通过该测定,我们检查了 OGT 超螺旋四肽重复 (TPR) 结构域内的保守天冬酰胺梯对底物选择的贡献。当五个天冬酰胺发生突变时,OGT 保留了针对短肽的显着活性,但在微阵列上显示出蛋白质底物的有限糖基化。细胞提取物中蛋白质底物的O-GlcNAc酰化也大大减弱。我们得出的结论是,OGT 通过将其结合到靠近催化结构域的 TPR 腔中的天冬酰胺梯来识别其大部分底物。































 京公网安备 11010802027423号
京公网安备 11010802027423号