当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Plasticity of the Carbohydrate Recognition Domain Dictates the Exquisite Mechanism of Binding of Human Macrophage Galactose-Type Lectin.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-10-01 , DOI: 10.1002/chem.201902780 Ana Diniz 1 , Helena Coelho 1, 2, 3 , Jorge S Dias 1 , Sandra J van Vliet 4 , Jesús Jiménez-Barbero 2, 3, 5 , Francisco Corzana 6 , Eurico J Cabrita 1 , Filipa Marcelo 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-10-01 , DOI: 10.1002/chem.201902780 Ana Diniz 1 , Helena Coelho 1, 2, 3 , Jorge S Dias 1 , Sandra J van Vliet 4 , Jesús Jiménez-Barbero 2, 3, 5 , Francisco Corzana 6 , Eurico J Cabrita 1 , Filipa Marcelo 1
Affiliation
|
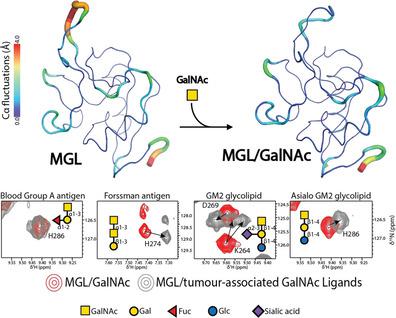
The human macrophage galactose-type lectin (MGL), expressed on macrophages and dendritic cells (DCs), modulates distinct immune cell responses by recognizing N-acetylgalactosamine (GalNAc) containing structures present on pathogens, self-glycoproteins, and tumor cells. Herein, NMR spectroscopy and molecular dynamics (MD) simulations were used to investigate the structural preferences of MGL against different GalNAc-containing structures derived from the blood group A antigen, the Forssman antigen, and the GM2 glycolipid. NMR spectroscopic analysis of the MGL carbohydrate recognition domain (MGL-CRD, C181-H316) in the absence and presence of methyl α-GalNAc (α-MeGalNAc), a simple monosaccharide, shows that the MGL-CRD is highly dynamic and its structure is strongly altered upon ligand binding. This plasticity of the MGL-CRD structure explains the ability of MGL to accommodate different GalNAc-containing molecules. However, key differences are observed in the recognition process depending on whether the GalNAc is part of the blood group A antigen, the Forssman antigen, or GM2-derived structures. These results are in accordance with molecular dynamics simulations that suggest the existence of a distinct MGL binding mechanism depending on the context of GalNAc moiety presentation. These results afford new perspectives for the rational design of GalNAc modifications that fine tune MGL immune responses in distinct biological contexts, especially in malignancy.
中文翻译:

碳水化合物识别域的可塑性决定了人类巨噬细胞半乳糖型凝集素结合的精致机制。
在巨噬细胞和树突状细胞(DC)上表达的人类巨噬细胞半乳糖型凝集素(MGL)通过识别病原体,自身糖蛋白和肿瘤细胞上存在的N-乙酰半乳糖胺(GalNAc)来调节不同的免疫细胞反应。本文中,使用NMR光谱和分子动力学(MD)模拟来研究MGL对源自血型A抗原,Forssman抗原和GM2糖脂的不同含GalNAc的结构的结构偏好。在不存在和存在简单单糖甲基α-GalNAc(α-MeGalNAc)的情况下,对MGL碳水化合物识别域(MGL-CRD,C181-H316)的NMR光谱分析表明,MGL-CRD具有高度的动态性和结构在配体结合后被强烈改变。MGL-CRD结构的这种可塑性解释了MGL适应不同的含GalNAc分子的能力。但是,根据GalNAc是A型血抗原,Forssman抗原还是GM2衍生结构的一部分,在识别过程中会观察到关键差异。这些结果与分子动力学模拟相符,分子动力学模拟表明,取决于GalNAc部分呈递的背景,存在独特的MGL结合机制。这些结果为合理设计GalNAc修饰提供了新的视角,这些修饰可以在不同的生物学环境中,特别是在恶性肿瘤中微调MGL免疫反应。根据GalNAc是A型血液抗原,Forssman抗原还是GM2衍生结构的一部分,在识别过程中会观察到关键差异。这些结果与分子动力学模拟相符,分子动力学模拟表明,取决于GalNAc部分呈递的背景,存在独特的MGL结合机制。这些结果为合理设计GalNAc修饰提供了新的视角,这些修饰可以在不同的生物学环境中,特别是在恶性肿瘤中微调MGL免疫反应。根据GalNAc是A型血液抗原,Forssman抗原还是GM2衍生结构的一部分,在识别过程中会观察到关键差异。这些结果与分子动力学模拟相符,分子动力学模拟表明,取决于GalNAc部分呈递的背景,存在独特的MGL结合机制。这些结果为合理设计GalNAc修饰提供了新的视角,这些修饰可以在不同的生物学环境中,特别是在恶性肿瘤中微调MGL免疫反应。
更新日期:2019-10-01
中文翻译:

碳水化合物识别域的可塑性决定了人类巨噬细胞半乳糖型凝集素结合的精致机制。
在巨噬细胞和树突状细胞(DC)上表达的人类巨噬细胞半乳糖型凝集素(MGL)通过识别病原体,自身糖蛋白和肿瘤细胞上存在的N-乙酰半乳糖胺(GalNAc)来调节不同的免疫细胞反应。本文中,使用NMR光谱和分子动力学(MD)模拟来研究MGL对源自血型A抗原,Forssman抗原和GM2糖脂的不同含GalNAc的结构的结构偏好。在不存在和存在简单单糖甲基α-GalNAc(α-MeGalNAc)的情况下,对MGL碳水化合物识别域(MGL-CRD,C181-H316)的NMR光谱分析表明,MGL-CRD具有高度的动态性和结构在配体结合后被强烈改变。MGL-CRD结构的这种可塑性解释了MGL适应不同的含GalNAc分子的能力。但是,根据GalNAc是A型血抗原,Forssman抗原还是GM2衍生结构的一部分,在识别过程中会观察到关键差异。这些结果与分子动力学模拟相符,分子动力学模拟表明,取决于GalNAc部分呈递的背景,存在独特的MGL结合机制。这些结果为合理设计GalNAc修饰提供了新的视角,这些修饰可以在不同的生物学环境中,特别是在恶性肿瘤中微调MGL免疫反应。根据GalNAc是A型血液抗原,Forssman抗原还是GM2衍生结构的一部分,在识别过程中会观察到关键差异。这些结果与分子动力学模拟相符,分子动力学模拟表明,取决于GalNAc部分呈递的背景,存在独特的MGL结合机制。这些结果为合理设计GalNAc修饰提供了新的视角,这些修饰可以在不同的生物学环境中,特别是在恶性肿瘤中微调MGL免疫反应。根据GalNAc是A型血液抗原,Forssman抗原还是GM2衍生结构的一部分,在识别过程中会观察到关键差异。这些结果与分子动力学模拟相符,分子动力学模拟表明,取决于GalNAc部分呈递的背景,存在独特的MGL结合机制。这些结果为合理设计GalNAc修饰提供了新的视角,这些修饰可以在不同的生物学环境中,特别是在恶性肿瘤中微调MGL免疫反应。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号