当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Voltage-Switchable HCl Transport Enabled by Lipid Headgroup-Transporter Interactions.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-08-30 , DOI: 10.1002/anie.201907466 Xin Wu 1 , Jennifer R Small 1, 2 , Alessio Cataldo 1, 3 , Anne M Withecombe 1 , Peter Turner 1 , Philip A Gale 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-08-30 , DOI: 10.1002/anie.201907466 Xin Wu 1 , Jennifer R Small 1, 2 , Alessio Cataldo 1, 3 , Anne M Withecombe 1 , Peter Turner 1 , Philip A Gale 1
Affiliation
|
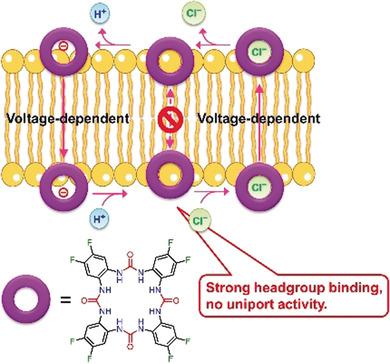
Synthetic anion transporters that facilitate transmembrane H+ /Cl- symport (cotransport) have anti-cancer potential due to their ability to neutralize pH gradients and inhibit autophagy in cells. However, compared to the natural product prodigiosin, synthetic anion transporters have low-to-modest H+ /Cl- symport activity and their mechanism of action remains less well understood. We report a chloride-selective tetraurea macrocycle that has a record-high H+ /Cl- symport activity similar to that of prodigiosin and most importantly demonstrates unprecedented voltage-switchable transport properties that are linked to the lack of uniport activity. By studying the anion binding affinity and transport mechanisms of four other anion transporters, we show that the lack of uniport and voltage-dependent H+ /Cl- symport originate from strong binding to phospholipid headgroups that hampers the diffusion of the free transporters through the membrane, leading to an unusual H+ /Cl- symport mechanism that involves only charged species. Our work provides important mechanistic insights into different classes of anion transporters and a new approach to achieve voltage-switchability in artificial membrane transport systems.
中文翻译:

脂质头基-转运体相互作用可实现电压可切换的HCl转运。
促进跨膜H + / Cl-共迁移(共转运)的合成阴离子转运蛋白具有中和pH梯度和抑制细胞自噬的能力,因此具有抗癌潜力。但是,与天然产物prodigiosin相比,合成阴离子转运蛋白具有低至中等的H + / Cl-交换活性,其作用机理仍不太清楚。我们报告了一个氯化物选择性的四脲大环化合物,其具有与prodigiosin相似的创纪录的高H + / Cl-交换活性,最重要的是证明了空缺的电压可交换的运输特性,这与缺乏单端口活性有关。通过研究其他四个阴离子转运蛋白的阴离子结合亲和力和转运机理,我们表明缺乏单端口和电压依赖性的H + / Cl-共迁移源于与磷脂头基的强结合,从而阻碍了自由转运蛋白通过膜的扩散,从而导致异常的H + / Cl-共迁移机制仅涉及带电物种。我们的工作为不同类别的阴离子转运蛋白提供了重要的机械见解,并为在人工膜转运系统中实现电压切换提供了一种新方法。
更新日期:2019-08-30
中文翻译:

脂质头基-转运体相互作用可实现电压可切换的HCl转运。
促进跨膜H + / Cl-共迁移(共转运)的合成阴离子转运蛋白具有中和pH梯度和抑制细胞自噬的能力,因此具有抗癌潜力。但是,与天然产物prodigiosin相比,合成阴离子转运蛋白具有低至中等的H + / Cl-交换活性,其作用机理仍不太清楚。我们报告了一个氯化物选择性的四脲大环化合物,其具有与prodigiosin相似的创纪录的高H + / Cl-交换活性,最重要的是证明了空缺的电压可交换的运输特性,这与缺乏单端口活性有关。通过研究其他四个阴离子转运蛋白的阴离子结合亲和力和转运机理,我们表明缺乏单端口和电压依赖性的H + / Cl-共迁移源于与磷脂头基的强结合,从而阻碍了自由转运蛋白通过膜的扩散,从而导致异常的H + / Cl-共迁移机制仅涉及带电物种。我们的工作为不同类别的阴离子转运蛋白提供了重要的机械见解,并为在人工膜转运系统中实现电压切换提供了一种新方法。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号