当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxazoline-/Copper-Catalyzed Alkoxyl Radical Generation: Solvent-Switched to Access 3a,3a'-Bisfuroindoline and 3-Alkoxyl Furoindoline.
Organic Letters ( IF 4.9 ) Pub Date : 2019-08-09 00:00:00 , DOI: 10.1021/acs.orglett.9b02394 Hai Ren 1, 2 , Jun-Rong Song 1, 2 , Zhi-Yao Li 1, 2 , Wei-Dong Pan 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2019-08-09 00:00:00 , DOI: 10.1021/acs.orglett.9b02394 Hai Ren 1, 2 , Jun-Rong Song 1, 2 , Zhi-Yao Li 1, 2 , Wei-Dong Pan 1, 2
Affiliation
|
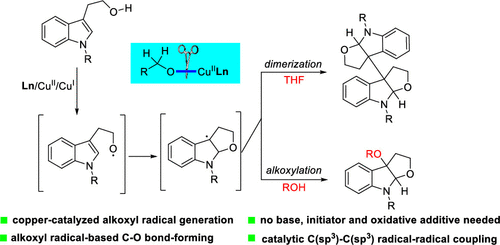
We report the first example of oxazoline-/copper-catalyzed alcohol oxidation to generate the alkoxyl radical under additive-free conditions. The resulting alkoxyl radical addition to alkene enables useful C-O bond-forming and selective C(sp3)-C(sp3) radical-radical dimerization/radical-trapping reactions, providing direct access to the 3a,3a'-bisfuro[2,3-b]indoline scaffold for the first time and a wide range of 3-alkoxyl furoindolines with high efficiency.
中文翻译:

恶唑啉/铜催化的烷氧基自由基生成:溶剂转换为访问3a,3a'-双呋喃二氢吲哚和3-烷氧基呋喃二氢吲哚。
我们报道了在无添加剂条件下恶唑啉/铜催化的醇氧化生成烷氧基自由基的第一个例子。生成的烷氧基自由基加成到烯烃中后,可形成有用的CO键,并选择性地进行C(sp3)-C(sp3)自由基-自由基二聚/自由基捕获反应,从而直接进入3a,3a'-bisfuro [2,3- b]首次使用吲哚啉骨架和高效的多种3-烷氧基呋喃二氢吲哚。
更新日期:2019-08-09
中文翻译:

恶唑啉/铜催化的烷氧基自由基生成:溶剂转换为访问3a,3a'-双呋喃二氢吲哚和3-烷氧基呋喃二氢吲哚。
我们报道了在无添加剂条件下恶唑啉/铜催化的醇氧化生成烷氧基自由基的第一个例子。生成的烷氧基自由基加成到烯烃中后,可形成有用的CO键,并选择性地进行C(sp3)-C(sp3)自由基-自由基二聚/自由基捕获反应,从而直接进入3a,3a'-bisfuro [2,3- b]首次使用吲哚啉骨架和高效的多种3-烷氧基呋喃二氢吲哚。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号