当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stable and Unstable Diglyme-Based Electrolytes for Batteries with Sodium or Graphite as Electrode
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-08-09 00:00:00 , DOI: 10.1021/acsami.9b06760 Mustafa Goktas 1, 2 , Christoph Bolli 3 , Johannes Buchheim 1, 2 , Erik J. Berg 4 , Petr Novák 3 , Francisco Bonilla 5 , Teófilo Rojo 5 , Shinichi Komaba 6 , Kei Kubota 6 , Philipp Adelhelm 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-08-09 00:00:00 , DOI: 10.1021/acsami.9b06760 Mustafa Goktas 1, 2 , Christoph Bolli 3 , Johannes Buchheim 1, 2 , Erik J. Berg 4 , Petr Novák 3 , Francisco Bonilla 5 , Teófilo Rojo 5 , Shinichi Komaba 6 , Kei Kubota 6 , Philipp Adelhelm 1, 2
Affiliation
|
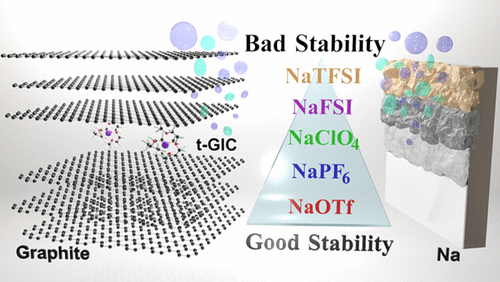
We study the stability of several diglyme-based electrolytes in sodium|sodium and sodium|graphite cells. The electrolyte behavior for different conductive salts [sodium trifluoromethanesulfonate (NaOTf), NaPF6, NaClO4, bis(fluorosulfonyl)imide (NaFSI), and sodium bis(trifluoromethanesulfonyl)imide (NaTFSI)] is compared and, in some cases, considerable differences are identified. Side reactions are studied with a variety of methods, including X-ray diffraction, scanning electron microscopy, transmission electron microscopy, online electrochemical mass spectrometry, and in situ electrochemical dilatometry. For Na|Na symmetric cells as well as for Na|graphite cells, we find that NaOTf and NaPF6 are the preferred salts followed by NaClO4 and NaFSI, as the latter two lead to more side reactions and increasing impedance. NaTFSI shows the worst performance leading to poor Coulombic efficiency and cycle life. In this case, excessive side reactions lead also to a strong increase in electrode thickness during cycling. In a qualitative order, the suitability of the conductive salts can be ranked as follows: NaOTf ≥ NaPF6 > NaClO4 > NaFSI ≫ NaTFSI. Our results also explain two recent, seemingly conflicting findings on the degree of solid electrolyte interphase formation on graphite electrodes in sodium-ion batteries [Maibach, J. ; ACS Appl. Mater. Interfaces 2017, 9, 12373−12381; Goktas, M. ; Adv. Energy Mater. 2018, 8, 1702724 ]. The contradictory findings are due to the different conductive salts used in both studies.
中文翻译:

以钠或石墨为电极的稳定且不稳定的基于二甘醇二甲醚的电解液
我们研究了钠和石墨钠电池中几种基于二甘醇二甲醚的电解质的稳定性。比较了不同导电盐[三氟甲磺酸钠(NaOTf),NaPF 6,NaClO 4,双(氟磺酰基)酰亚胺(NaFSI)和双(三氟甲磺酰基)酰亚胺钠(NaTFSI)]的电解质行为,在某些情况下,存在很大差异被识别。用多种方法研究了副反应,包括X射线衍射,扫描电子显微镜,透射电子显微镜,在线电化学质谱和原位电化学膨胀法。对于Na | Na对称电池以及Na |石墨电池,我们发现NaOTf和NaPF 6是优选的盐,其次是NaClO 4和NaFSI,因为后两者会导致更多的副反应并增加阻抗。NaTFSI显示最差的性能,导致库仑效率和循环寿命差。在这种情况下,过度的副反应还导致循环期间电极厚度的强烈增加。按定性顺序,导电盐的适用性可按以下等级排序:NaOTf≥NaPF 6 > NaClO 4 > NaFSI> NaTFSI。我们的结果还解释了关于钠离子电池中石墨电极上固体电解质中间相形成程度的两个最近看似矛盾的发现[迈巴赫,J。 ; ACS应用 母校 接口 2017,9,12373-12381;哥达斯,M。 ; 进阶 能源硕士。 2018,8,1702724 ]。矛盾的发现是由于两项研究中使用的导电盐不同。
更新日期:2019-08-09
中文翻译:

以钠或石墨为电极的稳定且不稳定的基于二甘醇二甲醚的电解液
我们研究了钠和石墨钠电池中几种基于二甘醇二甲醚的电解质的稳定性。比较了不同导电盐[三氟甲磺酸钠(NaOTf),NaPF 6,NaClO 4,双(氟磺酰基)酰亚胺(NaFSI)和双(三氟甲磺酰基)酰亚胺钠(NaTFSI)]的电解质行为,在某些情况下,存在很大差异被识别。用多种方法研究了副反应,包括X射线衍射,扫描电子显微镜,透射电子显微镜,在线电化学质谱和原位电化学膨胀法。对于Na | Na对称电池以及Na |石墨电池,我们发现NaOTf和NaPF 6是优选的盐,其次是NaClO 4和NaFSI,因为后两者会导致更多的副反应并增加阻抗。NaTFSI显示最差的性能,导致库仑效率和循环寿命差。在这种情况下,过度的副反应还导致循环期间电极厚度的强烈增加。按定性顺序,导电盐的适用性可按以下等级排序:NaOTf≥NaPF 6 > NaClO 4 > NaFSI> NaTFSI。我们的结果还解释了关于钠离子电池中石墨电极上固体电解质中间相形成程度的两个最近看似矛盾的发现[


















































 京公网安备 11010802027423号
京公网安备 11010802027423号