当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CDK7 inhibitor THZ1 inhibits MCL1 synthesis and drives cholangiocarcinoma apoptosis in combination with BCL2/BCL-XL inhibitor ABT-263.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-08-09 , DOI: 10.1038/s41419-019-1831-7
Tianlu Huang 1 , Xiwei Ding 1 , Guifang Xu 1 , Gang Chen 2 , Yu Cao 1 , Chunyan Peng 1 , Shanshan Shen 1 , Ying Lv 1 , Lei Wang 1 , Xiaoping Zou 1
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-08-09 , DOI: 10.1038/s41419-019-1831-7
Tianlu Huang 1 , Xiwei Ding 1 , Guifang Xu 1 , Gang Chen 2 , Yu Cao 1 , Chunyan Peng 1 , Shanshan Shen 1 , Ying Lv 1 , Lei Wang 1 , Xiaoping Zou 1
Affiliation
|
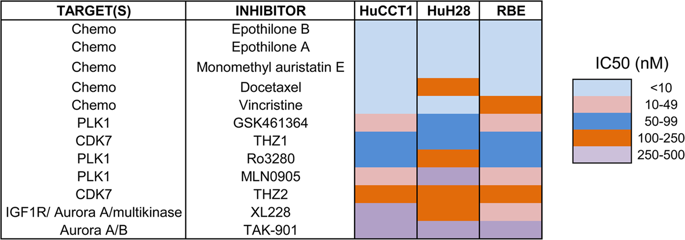
Cholangiocarcinoma (CCA) is a fatal disease without effective targeted therapy. We screened a small-molecule library of 116 inhibitors targeting different targets of the cell cycle and discovered several kinases, including Cyclin-dependent kinase 7 (CDK7) as vulnerabilities in CCA. Analysis of multiple CCA data sets demonstrated that CDK7 was overexpressed in CCA tissues. Further studies demonstrated that CDK7 inhibitor THZ1 inhibited cell viability and induced apoptosis in CCA cells. We also showed that THZ1 inhibited CCA cell growth in a xenograft model. RNA-sequencing followed by Gene ontology analysis showed a striking impact of THZ1 on DNA-templated transcriptional programs. THZ1 downregulated CDK7-mediated phosphorylation of RNA polymerase II, indicative of transcriptional inhibition. A number of oncogenic transcription factors and survival proteins, like MCL1, FOSL1, and RUNX1, were repressed by THZ1. MCL1, one of the antiapoptotic BCL2 family members, was significantly inhibited upon THZ1 treatment. Accordingly, combining THZ1 with a BCL2/BCL-XL inhibitor ABT-263 synergized in impairing cell growth and driving apoptosis. Our results demonstrate CDK7 as a potential target in treating CCA. Combinations of CDK7 inhibition and BCL2/BCL-XL inhibition may offer a novel therapeutic strategy for CCA.
中文翻译:

CDK7抑制剂THZ1与BCL2 / BCL-XL抑制剂ABT-263结合使用可抑制MCL1的合成并驱动胆管癌的细胞凋亡。
胆管癌(CCA)是一种致命的疾病,没有有效的靶向治疗。我们筛选了针对细胞周期不同靶点的116种抑制剂的小分子文库,并发现了几种激酶,包括Cyclin依赖性激酶7(CDK7)作为CCA中的脆弱性。对多个CCA数据集的分析表明,CDK7在CCA组织中过表达。进一步的研究表明,CDK7抑制剂THZ1抑制细胞活力并诱导CCA细胞凋亡。我们还显示在异种移植模型中,THZ1抑制了CCA细胞的生长。RNA测序,然后进行基因本体分析,显示THZ1对DNA模板转录程序的惊人影响。THZ1下调CDK7介导的RNA聚合酶II的磷酸化,表明转录抑制。THZ1抑制了许多致癌转录因子和生存蛋白,例如MCL1,FOSL1和RUNX1。抗凋亡BCL2家族成员之一MCL1在THZ1治疗后被显着抑制。因此,将THZ1与BCL2 / BCL-XL抑制剂ABT-263联合使用可减弱细胞生长和驱动细胞凋亡。我们的结果证明CDK7是治疗CCA的潜在靶标。CDK7抑制和BCL2 / BCL-XL抑制的组合可能为CCA提供新的治疗策略。我们的结果证明CDK7是治疗CCA的潜在靶标。CDK7抑制和BCL2 / BCL-XL抑制的组合可能为CCA提供新的治疗策略。我们的结果证明CDK7是治疗CCA的潜在靶标。CDK7抑制和BCL2 / BCL-XL抑制的组合可能为CCA提供新的治疗策略。
更新日期:2019-08-09
中文翻译:

CDK7抑制剂THZ1与BCL2 / BCL-XL抑制剂ABT-263结合使用可抑制MCL1的合成并驱动胆管癌的细胞凋亡。
胆管癌(CCA)是一种致命的疾病,没有有效的靶向治疗。我们筛选了针对细胞周期不同靶点的116种抑制剂的小分子文库,并发现了几种激酶,包括Cyclin依赖性激酶7(CDK7)作为CCA中的脆弱性。对多个CCA数据集的分析表明,CDK7在CCA组织中过表达。进一步的研究表明,CDK7抑制剂THZ1抑制细胞活力并诱导CCA细胞凋亡。我们还显示在异种移植模型中,THZ1抑制了CCA细胞的生长。RNA测序,然后进行基因本体分析,显示THZ1对DNA模板转录程序的惊人影响。THZ1下调CDK7介导的RNA聚合酶II的磷酸化,表明转录抑制。THZ1抑制了许多致癌转录因子和生存蛋白,例如MCL1,FOSL1和RUNX1。抗凋亡BCL2家族成员之一MCL1在THZ1治疗后被显着抑制。因此,将THZ1与BCL2 / BCL-XL抑制剂ABT-263联合使用可减弱细胞生长和驱动细胞凋亡。我们的结果证明CDK7是治疗CCA的潜在靶标。CDK7抑制和BCL2 / BCL-XL抑制的组合可能为CCA提供新的治疗策略。我们的结果证明CDK7是治疗CCA的潜在靶标。CDK7抑制和BCL2 / BCL-XL抑制的组合可能为CCA提供新的治疗策略。我们的结果证明CDK7是治疗CCA的潜在靶标。CDK7抑制和BCL2 / BCL-XL抑制的组合可能为CCA提供新的治疗策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号