当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photothermal Therapy Nanomaterials Boosting Transformation of Fe(III) into Fe(II) in Tumor Cells for Highly Improving Chemodynamic Therapy.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-08-20 , DOI: 10.1021/acsami.9b11291 Xuan Nie 1 , Lei Xia 1 , Hai-Li Wang 1 , Guang Chen 1 , Bin Wu 1 , Tian-You Zeng 1 , Chun-Yan Hong 1 , Long-Hai Wang 1 , Ye-Zi You 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-08-20 , DOI: 10.1021/acsami.9b11291 Xuan Nie 1 , Lei Xia 1 , Hai-Li Wang 1 , Guang Chen 1 , Bin Wu 1 , Tian-You Zeng 1 , Chun-Yan Hong 1 , Long-Hai Wang 1 , Ye-Zi You 1
Affiliation
|
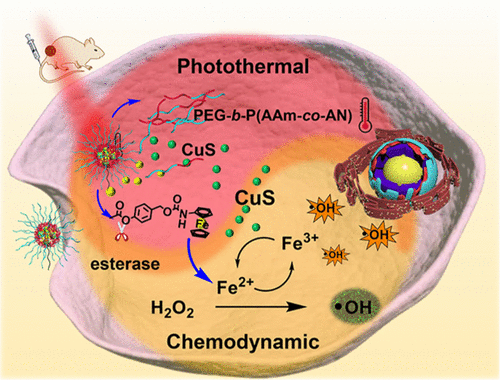
Chemodynamic therapy based on Fe2+-catalyzed Fenton reaction holds great promise in cancer treatment. However, low-produced hydroxyl radicals in tumor cells constitute its severe challenges because of the fact that Fe2+ with high catalytic activity could be easily oxidized into Fe3+ with low catalytic activity, greatly lowering Fenton reaction efficacy. Here, we codeliver CuS with the iron-containing prodrug into tumor cells. In tumor cells, the overproduced esterase could cleave the phenolic ester bond in the prodrug to release Fe2+, activating Fenton reaction to produce the hydroxyl radical. Meanwhile, CuS could act as a nanocatalyst for continuously catalyzing the regeneration of high-active Fe2+ from low-active Fe3+ to produce enough hydroxyl radicals to efficiently kill tumor cells as well as a photothermal therapy agent for generating hyperthermia for thermal ablation of tumor cells upon NIR irradiation. The results have exhibited that the approach of photothermal therapy nanomaterials boosting transformation of Fe3+ into Fe2+ in tumor cells can highly improve Fenton reaction for efficient chemodynamic therapy. This strategy was demonstrated to have an excellent antitumor activity both in vitro and in vivo, which provides an innovative perspective to Fenton reaction-based chemodynamic therapy.
中文翻译:

光热疗法纳米材料可促进肿瘤细胞中Fe(III)向Fe(II)的转化,从而高度改善化学动力疗法。
基于Fe2 +催化的Fenton反应的化学动力学疗法在癌症治疗中具有广阔的前景。然而,由于具有高催化活性的Fe 2+容易被氧化成具有低催化活性的Fe 3+,因此肿瘤细胞中低产的羟基自由基构成了其严峻的挑战,大大降低了芬顿反应的功效。在这里,我们将含铁的前药将CuS编码到肿瘤细胞中。在肿瘤细胞中,过量产生的酯酶可能会裂解前药中的酚酯键以释放Fe2 +,从而激活Fenton反应产生羟基自由基。同时,CuS可以充当纳米催化剂,持续催化高活性Fe2 +从低活性Fe3 +的再生,产生足够的羟基自由基以有效杀死肿瘤细胞,还可以用作光热治疗剂以产生高热,从而在NIR照射下对肿瘤细胞进行热消融。结果表明,光热疗法纳米材料促进肿瘤细胞中Fe3 +转化为Fe2 +的方法可以极大地改善Fenton反应,从而实现有效的化学动力学治疗。事实证明,该策略在体外和体内均具有出色的抗肿瘤活性,这为基于Fenton反应的化学动力学治疗提供了创新的视角。结果表明,光热疗法纳米材料促进肿瘤细胞中Fe3 +转化为Fe2 +的方法可以极大地改善Fenton反应,从而实现有效的化学动力学治疗。事实证明,该策略在体外和体内均具有出色的抗肿瘤活性,这为基于Fenton反应的化学动力学治疗提供了创新的视角。结果表明,光热疗法纳米材料促进肿瘤细胞中Fe3 +转化为Fe2 +的方法可以极大地改善Fenton反应,从而实现有效的化学动力学治疗。事实证明,该策略在体外和体内均具有出色的抗肿瘤活性,这为基于Fenton反应的化学动力学治疗提供了创新的视角。
更新日期:2019-08-08
中文翻译:

光热疗法纳米材料可促进肿瘤细胞中Fe(III)向Fe(II)的转化,从而高度改善化学动力疗法。
基于Fe2 +催化的Fenton反应的化学动力学疗法在癌症治疗中具有广阔的前景。然而,由于具有高催化活性的Fe 2+容易被氧化成具有低催化活性的Fe 3+,因此肿瘤细胞中低产的羟基自由基构成了其严峻的挑战,大大降低了芬顿反应的功效。在这里,我们将含铁的前药将CuS编码到肿瘤细胞中。在肿瘤细胞中,过量产生的酯酶可能会裂解前药中的酚酯键以释放Fe2 +,从而激活Fenton反应产生羟基自由基。同时,CuS可以充当纳米催化剂,持续催化高活性Fe2 +从低活性Fe3 +的再生,产生足够的羟基自由基以有效杀死肿瘤细胞,还可以用作光热治疗剂以产生高热,从而在NIR照射下对肿瘤细胞进行热消融。结果表明,光热疗法纳米材料促进肿瘤细胞中Fe3 +转化为Fe2 +的方法可以极大地改善Fenton反应,从而实现有效的化学动力学治疗。事实证明,该策略在体外和体内均具有出色的抗肿瘤活性,这为基于Fenton反应的化学动力学治疗提供了创新的视角。结果表明,光热疗法纳米材料促进肿瘤细胞中Fe3 +转化为Fe2 +的方法可以极大地改善Fenton反应,从而实现有效的化学动力学治疗。事实证明,该策略在体外和体内均具有出色的抗肿瘤活性,这为基于Fenton反应的化学动力学治疗提供了创新的视角。结果表明,光热疗法纳米材料促进肿瘤细胞中Fe3 +转化为Fe2 +的方法可以极大地改善Fenton反应,从而实现有效的化学动力学治疗。事实证明,该策略在体外和体内均具有出色的抗肿瘤活性,这为基于Fenton反应的化学动力学治疗提供了创新的视角。































 京公网安备 11010802027423号
京公网安备 11010802027423号