当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Integrated Synthetic, Biophysical, and Computational Investigations of Covalent Inhibitors of Prolyl Oligopeptidase and Fibroblast Activation Protein α.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-08-22 , DOI: 10.1021/acs.jmedchem.9b00642 Jessica Plescia 1 , Stéphane De Cesco 1 , Mihai Burai Patrascu 1 , Jerry Kurian 1 , Justin Di Trani 1 , Caroline Dufresne 1 , Alexander S Wahba 1 , Naëla Janmamode 1 , Anthony K Mittermaier 1 , Nicolas Moitessier 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-08-22 , DOI: 10.1021/acs.jmedchem.9b00642 Jessica Plescia 1 , Stéphane De Cesco 1 , Mihai Burai Patrascu 1 , Jerry Kurian 1 , Justin Di Trani 1 , Caroline Dufresne 1 , Alexander S Wahba 1 , Naëla Janmamode 1 , Anthony K Mittermaier 1 , Nicolas Moitessier 1
Affiliation
|
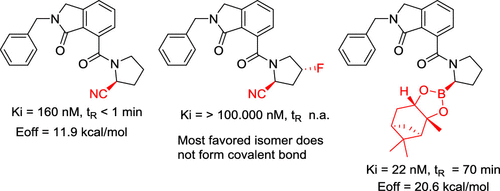
Over the past decade, there has been increasing interest in covalent inhibition as a drug design strategy. Our own interest in the development of prolyl oligopeptidase (POP) and fibroblast activation protein α (FAP) covalent inhibitors has led us to question whether these two serine proteases were equal in terms of their reactivity toward electrophilic warheads. To streamline such investigations, we exploited both computational and experimental methods to investigate the influence of different reactive groups on both potency and binding kinetics using both our own series of POP inhibitors and others' discovered hits. A direct correlation between inhibitor reactivity and residence time was demonstrated through quantum mechanics methods and further supported by experimental studies. This computational method was also successfully applied to FAP, as an overview of known FAP inhibitors confirmed our computational predictions that more reactive warheads (e.g., boronic acids) must be employed to inhibit FAP than for POP.
中文翻译:

脯氨酰寡肽酶和成纤维细胞活化蛋白α的共价抑制剂的综合合成,生物物理和计算研究。
在过去的十年中,对共价抑制作为药物设计策略的兴趣日益增加。我们对脯氨酰寡肽酶(POP)和成纤维细胞活化蛋白α(FAP)共价抑制剂的开发感兴趣,这使我们质疑这两种丝氨酸蛋白酶对亲电子战斗部的反应性是否相等。为了简化此类研究,我们利用计算和实验方法,使用我们自己的一系列POP抑制剂和其他发现的命中物,研究了不同反应性基团对效价和结合动力学的影响。通过量子力学方法证明了抑制剂反应性和停留时间之间的直接关系,并得到了实验研究的进一步支持。这种计算方法也成功地应用于FAP,
更新日期:2019-08-08
中文翻译:

脯氨酰寡肽酶和成纤维细胞活化蛋白α的共价抑制剂的综合合成,生物物理和计算研究。
在过去的十年中,对共价抑制作为药物设计策略的兴趣日益增加。我们对脯氨酰寡肽酶(POP)和成纤维细胞活化蛋白α(FAP)共价抑制剂的开发感兴趣,这使我们质疑这两种丝氨酸蛋白酶对亲电子战斗部的反应性是否相等。为了简化此类研究,我们利用计算和实验方法,使用我们自己的一系列POP抑制剂和其他发现的命中物,研究了不同反应性基团对效价和结合动力学的影响。通过量子力学方法证明了抑制剂反应性和停留时间之间的直接关系,并得到了实验研究的进一步支持。这种计算方法也成功地应用于FAP,


















































 京公网安备 11010802027423号
京公网安备 11010802027423号