当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of β3-Amino Esters by Iridium-Catalyzed Asymmetric Allylic Alkylation Reaction
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-08-07 00:00:00 , DOI: 10.1021/acs.oprd.9b00280 Chao-Guo Cao 1 , Bin He 1 , Zhengyan Fu 1 , Dawen Niu 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-08-07 00:00:00 , DOI: 10.1021/acs.oprd.9b00280 Chao-Guo Cao 1 , Bin He 1 , Zhengyan Fu 1 , Dawen Niu 1
Affiliation
|
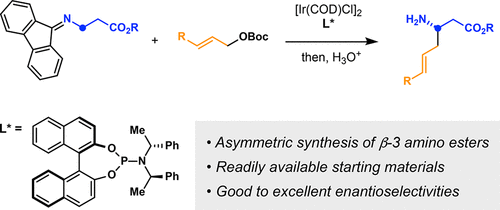
A method to prepare chiral β3-amino esters from methyl 3-aminopropanoate was described. This method capitalized on a sequence involving an Ir-catalyzed asymmetric allylation of 2-azaallyl anions and a 2-aza-Cope rearrangement. β3-Amino esters containing a versatile alkene group were prepared.
中文翻译:

β的合成3通过铱催化不对称烯丙基烷基化反应氨基酯
的方法制备的手性β 3个选自甲基-3-氨基丙氨基酯进行了说明。该方法利用了涉及2-氮杂烯丙基阴离子的Ir催化的不对称烯丙基化和2-氮杂-Cope重排的序列。β 3个制备含有一种多用途的烯烃基团氨基酯。
更新日期:2019-08-07
中文翻译:

β的合成3通过铱催化不对称烯丙基烷基化反应氨基酯
的方法制备的手性β 3个选自甲基-3-氨基丙氨基酯进行了说明。该方法利用了涉及2-氮杂烯丙基阴离子的Ir催化的不对称烯丙基化和2-氮杂-Cope重排的序列。β 3个制备含有一种多用途的烯烃基团氨基酯。































 京公网安备 11010802027423号
京公网安备 11010802027423号