Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pol II phosphorylation regulates a switch between transcriptional and splicing condensates
Nature ( IF 50.5 ) Pub Date : 2019-08-01 , DOI: 10.1038/s41586-019-1464-0
Yang Eric Guo 1 , John C Manteiga 1, 2 , Jonathan E Henninger 1 , Benjamin R Sabari 1 , Alessandra Dall'Agnese 1 , Nancy M Hannett 1 , Jan-Hendrik Spille 3, 4 , Lena K Afeyan 1, 2 , Alicia V Zamudio 1, 2 , Krishna Shrinivas 5, 6 , Brian J Abraham 1, 7 , Ann Boija 1 , Tim-Michael Decker 8 , Jenna K Rimel 8 , Charli B Fant 8 , Tong Ihn Lee 1 , Ibrahim I Cisse 3 , Phillip A Sharp 2, 9 , Dylan J Taatjes 8 , Richard A Young 1, 2
Nature ( IF 50.5 ) Pub Date : 2019-08-01 , DOI: 10.1038/s41586-019-1464-0
Yang Eric Guo 1 , John C Manteiga 1, 2 , Jonathan E Henninger 1 , Benjamin R Sabari 1 , Alessandra Dall'Agnese 1 , Nancy M Hannett 1 , Jan-Hendrik Spille 3, 4 , Lena K Afeyan 1, 2 , Alicia V Zamudio 1, 2 , Krishna Shrinivas 5, 6 , Brian J Abraham 1, 7 , Ann Boija 1 , Tim-Michael Decker 8 , Jenna K Rimel 8 , Charli B Fant 8 , Tong Ihn Lee 1 , Ibrahim I Cisse 3 , Phillip A Sharp 2, 9 , Dylan J Taatjes 8 , Richard A Young 1, 2
Affiliation
|
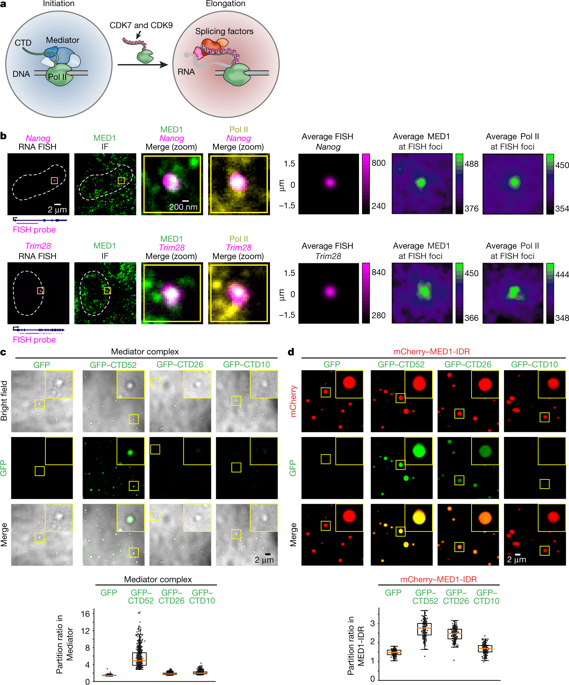
The synthesis of pre-mRNA by RNA polymerase II (Pol II) involves the formation of a transcription initiation complex, and a transition to an elongation complex1–4. The large subunit of Pol II contains an intrinsically disordered C-terminal domain that is phosphorylated by cyclin-dependent kinases during the transition from initiation to elongation, thus influencing the interaction of the C-terminal domain with different components of the initiation or the RNA-splicing apparatus5,6. Recent observations suggest that this model provides only a partial picture of the effects of phosphorylation of the C-terminal domain7–12. Both the transcription-initiation machinery and the splicing machinery can form phase-separated condensates that contain large numbers of component molecules: hundreds of molecules of Pol II and mediator are concentrated in condensates at super-enhancers7,8, and large numbers of splicing factors are concentrated in nuclear speckles, some of which occur at highly active transcription sites9–12. Here we investigate whether the phosphorylation of the Pol II C-terminal domain regulates the incorporation of Pol II into phase-separated condensates that are associated with transcription initiation and splicing. We find that the hypophosphorylated C-terminal domain of Pol II is incorporated into mediator condensates and that phosphorylation by regulatory cyclin-dependent kinases reduces this incorporation. We also find that the hyperphosphorylated C-terminal domain is preferentially incorporated into condensates that are formed by splicing factors. These results suggest that phosphorylation of the Pol II C-terminal domain drives an exchange from condensates that are involved in transcription initiation to those that are involved in RNA processing, and implicates phosphorylation as a mechanism that regulates condensate preference.RNA polymerase II with a hypophosphorylated C-terminal domain preferentially incorporates into mediator condensates, and with a hyperphosphorylated C-terminal domain into splicing-factor condensates, revealing phosphorylation as a regulatory mechanism in condensate preference.
中文翻译:

Pol II 磷酸化调节转录和剪接缩合物之间的转换
RNA 聚合酶 II (Pol II) 合成前 mRNA 涉及转录起始复合物的形成,以及向延伸复合物的转变1-4。 Pol II 的大亚基含有一个本质上无序的 C 端结构域,在从起始到延伸的过渡过程中,该结构域被细胞周期蛋白依赖性激酶磷酸化,从而影响 C 端结构域与起始或 RNA 的不同成分的相互作用。拼接装置5,6。最近的观察表明,该模型仅提供了 C 末端结构域磷酸化影响的部分图片7-12。转录起始机制和剪接机制都可以形成包含大量组成分子的相分离凝聚物:数百个Pol II和介体分子集中在超级增强子7,8处的凝聚物中,并且大量剪接因子集中在核斑点中,其中一些发生在高度活跃的转录位点9-12。在这里,我们研究 Pol II C 端结构域的磷酸化是否调节 Pol II 掺入与转录起始和剪接相关的相分离缩合物。我们发现 Pol II 的低磷酸化 C 端结构域被掺入介体缩合物中,并且调节性细胞周期蛋白依赖性激酶的磷酸化减少了这种掺入。我们还发现,过度磷酸化的 C 端结构域优先掺入剪接因子形成的缩合物中。 这些结果表明,Pol II C 末端结构域的磷酸化驱动了从参与转录起始的冷凝物到参与 RNA 加工的冷凝物的交换,并暗示磷酸化是调节冷凝物偏好的机制。RNA 聚合酶 II 具有低磷酸化C 端结构域优先掺入介体缩合物中,并且过度磷酸化的 C 端结构域掺入剪接因子缩合物中,揭示了磷酸化作为缩合物偏好中的调节机制。
更新日期:2019-08-01
中文翻译:

Pol II 磷酸化调节转录和剪接缩合物之间的转换
RNA 聚合酶 II (Pol II) 合成前 mRNA 涉及转录起始复合物的形成,以及向延伸复合物的转变1-4。 Pol II 的大亚基含有一个本质上无序的 C 端结构域,在从起始到延伸的过渡过程中,该结构域被细胞周期蛋白依赖性激酶磷酸化,从而影响 C 端结构域与起始或 RNA 的不同成分的相互作用。拼接装置5,6。最近的观察表明,该模型仅提供了 C 末端结构域磷酸化影响的部分图片7-12。转录起始机制和剪接机制都可以形成包含大量组成分子的相分离凝聚物:数百个Pol II和介体分子集中在超级增强子7,8处的凝聚物中,并且大量剪接因子集中在核斑点中,其中一些发生在高度活跃的转录位点9-12。在这里,我们研究 Pol II C 端结构域的磷酸化是否调节 Pol II 掺入与转录起始和剪接相关的相分离缩合物。我们发现 Pol II 的低磷酸化 C 端结构域被掺入介体缩合物中,并且调节性细胞周期蛋白依赖性激酶的磷酸化减少了这种掺入。我们还发现,过度磷酸化的 C 端结构域优先掺入剪接因子形成的缩合物中。 这些结果表明,Pol II C 末端结构域的磷酸化驱动了从参与转录起始的冷凝物到参与 RNA 加工的冷凝物的交换,并暗示磷酸化是调节冷凝物偏好的机制。RNA 聚合酶 II 具有低磷酸化C 端结构域优先掺入介体缩合物中,并且过度磷酸化的 C 端结构域掺入剪接因子缩合物中,揭示了磷酸化作为缩合物偏好中的调节机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号