当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insight into Propylene Epoxidation with H2O2 over Titanium Silicalite-1: Effects of Zeolite Confinement and Solvent
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-08-21 , DOI: 10.1021/acs.jpcb.9b04439 Xiaowa Nie , Xianxuan Ren , Xiaojing Ji , Yonggang Chen , Michael J. Janik , Xinwen Guo , Chunshan Song
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-08-21 , DOI: 10.1021/acs.jpcb.9b04439 Xiaowa Nie , Xianxuan Ren , Xiaojing Ji , Yonggang Chen , Michael J. Janik , Xinwen Guo , Chunshan Song
|
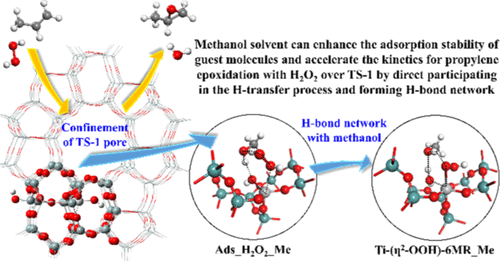
Density functional theory (DFT) calculations were performed to investigate the effects of zeolite confinement and solvent on propylene epoxidation with H2O2 over the titanium silicalite-1 (TS-1) catalyst. The 144T and 143T cluster models containing typical 10MR channels of TS-1 were constructed to represent the tripodal(2I) and Ti/defect sites. It was found that the confinement of the zeolite pore channel not only impacts the adsorption stability of guest molecules but also alters reaction barriers, as compared to the results obtained based on small cluster models. When dispersion corrections were considered, an enhancement of the adsorption stability of guest molecules was observed because of the important contribution from van der Waals interactions, especially for propylene adsorption. An explicit protic methanol molecule was introduced into the catalytic system to probe the influence of the solvent on propylene epoxidation, based on which a significant enhancement of CH3OH–H2O2 co-adsorption was obtained owing to H-bond formation. More importantly, the energy barrier for H2O2 dissociation was largely reduced by ∼13 kcal/mol because of the participation of the methanol in the H-transfer process and the formation of H-bond network, resulting in an alteration of the rate-limiting step. By comparison, adding an aprotic acetonitrile solvent did not have substantial effect on reaction path and kinetics. The calculation results clearly demonstrate the important role of the protic methanol solvent, which not only strengthens the adsorption of guest molecules but also promotes the kinetics for propylene epoxidation with H2O2 over TS-1 catalyst.
中文翻译:

Silicalite-1钛在H2O2上环氧丙烷环氧化的机理研究:沸石限制和溶剂的影响
进行了密度泛函理论(DFT)计算,以研究沸石限制剂和溶剂对H 2 O 2对丙烯环氧化的影响在钛硅沸石-1(TS-1)催化剂上。包含TS-1典型10MR通道的144T和143T群集模型被构建为代表三脚架(2I)和Ti /缺陷位点。与基于小簇模型的结果相比,发现沸石孔隙通道的限制不仅影响客体分子的吸附稳定性,而且改变了反应壁垒。当考虑色散校正时,由于范德华相互作用(尤其是丙烯吸附)起着重要作用,因此观察到客体分子的吸附稳定性得到了增强。一个明确的质子甲醇分子被引入到催化系统中,以探测溶剂对丙烯环氧化的影响,在此基础上,CH 3的显着增强由于形成了氢键,获得了OH–H 2 O 2共吸附。更重要的是,由于甲醇参与了H转移过程和H键网络的形成,H 2 O 2离解的能垒大大降低了约13 kcal / mol。 -限制步骤。相比之下,添加非质子乙腈溶剂对反应路径和动力学没有实质性影响。计算结果清楚地证明了质子甲醇溶剂的重要作用,它不仅增强了客体分子的吸附能力,而且还促进了TS-1催化剂上H 2 O 2对丙烯环氧化的动力学。
更新日期:2019-08-22
中文翻译:

Silicalite-1钛在H2O2上环氧丙烷环氧化的机理研究:沸石限制和溶剂的影响
进行了密度泛函理论(DFT)计算,以研究沸石限制剂和溶剂对H 2 O 2对丙烯环氧化的影响在钛硅沸石-1(TS-1)催化剂上。包含TS-1典型10MR通道的144T和143T群集模型被构建为代表三脚架(2I)和Ti /缺陷位点。与基于小簇模型的结果相比,发现沸石孔隙通道的限制不仅影响客体分子的吸附稳定性,而且改变了反应壁垒。当考虑色散校正时,由于范德华相互作用(尤其是丙烯吸附)起着重要作用,因此观察到客体分子的吸附稳定性得到了增强。一个明确的质子甲醇分子被引入到催化系统中,以探测溶剂对丙烯环氧化的影响,在此基础上,CH 3的显着增强由于形成了氢键,获得了OH–H 2 O 2共吸附。更重要的是,由于甲醇参与了H转移过程和H键网络的形成,H 2 O 2离解的能垒大大降低了约13 kcal / mol。 -限制步骤。相比之下,添加非质子乙腈溶剂对反应路径和动力学没有实质性影响。计算结果清楚地证明了质子甲醇溶剂的重要作用,它不仅增强了客体分子的吸附能力,而且还促进了TS-1催化剂上H 2 O 2对丙烯环氧化的动力学。

































 京公网安备 11010802027423号
京公网安备 11010802027423号