Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nascent Polypeptide Domain Topology and Elongation Rate Direct the Cotranslational Hierarchy of Hsp70 and TRiC/CCT.
Molecular Cell ( IF 14.5 ) Pub Date : 2019-08-07 , DOI: 10.1016/j.molcel.2019.06.036 Kevin C Stein 1 , Allison Kriel 1 , Judith Frydman 2
Molecular Cell ( IF 14.5 ) Pub Date : 2019-08-07 , DOI: 10.1016/j.molcel.2019.06.036 Kevin C Stein 1 , Allison Kriel 1 , Judith Frydman 2
Affiliation
|
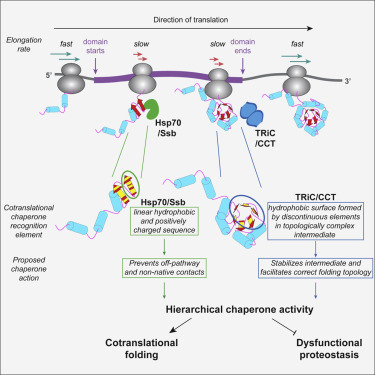
Cotranslational protein folding requires assistance from elaborate ribosome-associated chaperone networks. It remains unclear how the changing information in a growing nascent polypeptide dictates the recruitment of functionally distinct chaperones. Here, we used ribosome profiling to define the principles governing the cotranslational action of the chaperones TRiC/CCT and Hsp70/Ssb. We show that these chaperones are sequentially recruited to specific sites within domain-encoding regions of select nascent polypeptides. Hsp70 associates first, binding select sites throughout domains, whereas TRiC associates later, upon the emergence of nearly complete domains that expose an unprotected hydrophobic surface. This suggests that transient topological properties of nascent folding intermediates drive sequential chaperone association. Moreover, cotranslational recruitment of both TRiC and Hsp70 correlated with translation elongation slowdowns. We propose that the temporal modulation of the nascent chain structural landscape is coordinated with local elongation rates to regulate the hierarchical action of Hsp70 and TRiC for cotranslational folding.
中文翻译:

新生多肽域的拓扑结构和延伸率指导Hsp70和TRiC / CCT的共翻译层次。
共翻译蛋白折叠需要复杂的核糖体相关伴侣网络的协助。尚不清楚如何增长的新生多肽中的信息变化决定了功能上不同的伴侣的募集。在这里,我们使用核糖体图谱来定义控制分子伴侣TRiC / CCT和Hsp70 / Ssb的共翻译作用的原理。我们显示,这些分子伴侣被顺序招募到所选新生多肽的域编码区域内的特定位点。Hsp70首先结合,在整个域中结合选择位点,而TRiC随后结合,暴露出未保护的疏水表面的几乎完整的域出现。这表明新生的折叠中间体的瞬时拓扑性质驱动顺序的伴侣缔合。而且,TRiC和Hsp70的共翻译募集与翻译延伸减慢相关。我们建议新生链结构景观的时间调制与局部伸长率协调,以调节Hsp70和TRiC对共翻译折叠的分层作用。
更新日期:2019-08-07
中文翻译:

新生多肽域的拓扑结构和延伸率指导Hsp70和TRiC / CCT的共翻译层次。
共翻译蛋白折叠需要复杂的核糖体相关伴侣网络的协助。尚不清楚如何增长的新生多肽中的信息变化决定了功能上不同的伴侣的募集。在这里,我们使用核糖体图谱来定义控制分子伴侣TRiC / CCT和Hsp70 / Ssb的共翻译作用的原理。我们显示,这些分子伴侣被顺序招募到所选新生多肽的域编码区域内的特定位点。Hsp70首先结合,在整个域中结合选择位点,而TRiC随后结合,暴露出未保护的疏水表面的几乎完整的域出现。这表明新生的折叠中间体的瞬时拓扑性质驱动顺序的伴侣缔合。而且,TRiC和Hsp70的共翻译募集与翻译延伸减慢相关。我们建议新生链结构景观的时间调制与局部伸长率协调,以调节Hsp70和TRiC对共翻译折叠的分层作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号