当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A proton transfer mechanism along the PO4 anion chain in the [Zn(HPO4)(H2PO4)]2− coordination polymer†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2019-08-06 00:00:00 , DOI: 10.1039/c9cp04216d Hieu C. Dong 1, 2, 3, 4 , Hieu T. Hoang 1, 2, 3, 4 , Dinh Manh Tran 4, 5, 6, 7 , Thang B. Phan 1, 2, 3, 4 , Sareeya Bureekaew 8, 9, 10, 11, 12 , Yoshiyuki Kawazoe 13, 14, 15, 16 , Hung M. Le 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2019-08-06 00:00:00 , DOI: 10.1039/c9cp04216d Hieu C. Dong 1, 2, 3, 4 , Hieu T. Hoang 1, 2, 3, 4 , Dinh Manh Tran 4, 5, 6, 7 , Thang B. Phan 1, 2, 3, 4 , Sareeya Bureekaew 8, 9, 10, 11, 12 , Yoshiyuki Kawazoe 13, 14, 15, 16 , Hung M. Le 1, 2, 3, 4
Affiliation
|
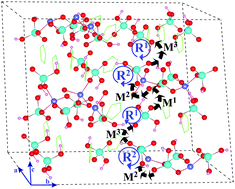
In this study, we revisit the proton transfer mechanism in [Zn(HPO4)(H2PO4)]2−, a coordination polymer possessing high proton conductivity. In a previous report [N. Phattharasupakun, J. Wutthiprom, S. Kaenket, Th. Maihom, J. Limtrakul, M. Probst, S. S. Nagarkar, S. Horike and M. Sawangphruk, Chem. Commun., 2017, 53, 11786–11789], it was hypothesized that protons could move along the ImH+ chain involving phosphate anions within the polymer structure, with energy barriers >1.3 eV. Adopting M06-2X calculations to examine the reaction pathway, we observe that it is much more favorable for H+ to move along a one-dimensional channel formed by HPO42− and H2PO4− anions. Within a unit cell, the proton hopping process can be divided into three elementary steps. For the forward proton transfer direction, the maximum energy barrier is only 0.04 eV, while that of the backward direction is 0.27 eV. Even though the barriers of the backward direction seem to outreach the barriers of the forward direction, both are still low in comparison with those reported in the literature. Moreover, we also point out the involvement of PO4 rotation during the proton transfer process. Activation energies of 0.37 eV and 0.15 eV are required for single steps of rotation of the phosphate anion. Both H+ translation (hopping) and rotation steps of PO4 anions simultaneously participate in the course of proton transfer in the coordination polymer.
中文翻译:

[Zn(HPO 4)(H 2 PO 4)] 2-配位聚合物† 沿PO 4阴离子链的质子转移机理
在这项研究中,我们将重新讨论[Zn(HPO 4)(H 2 PO 4)] 2-中的质子转移机理,该化合物是具有高质子传导性的配位聚合物。在以前的报告中[N. Phattharasupakun,J。Wutthiprom,S。Kaenket,Th。Maihom,J。Limtrakul,M。Probst,SS Nagarkar,S。Horike和M.Sawangphruk,Chem。公社 ,2017,53,11786-11789],有人推测质子可以沿着IMH移动+涉及在聚合物结构中磷酸根阴离子链,具有能垒> 1.3电子伏特。通过使用M06-2X计算来检查反应路径,我们发现它对H +更为有利沿着由HPO形成的一维信道移动4 2-和H 2 PO 4 -阴离子。在晶胞内,质子跳跃过程可分为三个基本步骤。对于正向质子传递方向,最大能垒仅为0.04 eV,而向后方向的最大能垒为0.27 eV。尽管后向的障碍似乎超过了前向的障碍,但与文献报道的相比,两者仍然较低。此外,我们还指出了质子转移过程中PO 4旋转的参与。磷酸根阴离子的单步旋转需要0.37 eV和0.15 eV的活化能。都H +PO 4阴离子的平移(跳跃)和旋转步骤同时参与配位聚合物中质子转移的过程。
更新日期:2019-08-06
中文翻译:

[Zn(HPO 4)(H 2 PO 4)] 2-配位聚合物† 沿PO 4阴离子链的质子转移机理
在这项研究中,我们将重新讨论[Zn(HPO 4)(H 2 PO 4)] 2-中的质子转移机理,该化合物是具有高质子传导性的配位聚合物。在以前的报告中[N. Phattharasupakun,J。Wutthiprom,S。Kaenket,Th。Maihom,J。Limtrakul,M。Probst,SS Nagarkar,S。Horike和M.Sawangphruk,Chem。公社 ,2017,53,11786-11789],有人推测质子可以沿着IMH移动+涉及在聚合物结构中磷酸根阴离子链,具有能垒> 1.3电子伏特。通过使用M06-2X计算来检查反应路径,我们发现它对H +更为有利沿着由HPO形成的一维信道移动4 2-和H 2 PO 4 -阴离子。在晶胞内,质子跳跃过程可分为三个基本步骤。对于正向质子传递方向,最大能垒仅为0.04 eV,而向后方向的最大能垒为0.27 eV。尽管后向的障碍似乎超过了前向的障碍,但与文献报道的相比,两者仍然较低。此外,我们还指出了质子转移过程中PO 4旋转的参与。磷酸根阴离子的单步旋转需要0.37 eV和0.15 eV的活化能。都H +PO 4阴离子的平移(跳跃)和旋转步骤同时参与配位聚合物中质子转移的过程。































 京公网安备 11010802027423号
京公网安备 11010802027423号