当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improving Cytotoxicity by Changing a Linker from Diphenylether to Diphenylmethane and now to Phenylene in Binuclear Dithiocarbamate Complexes: Synthesis and Cytotoxicity Study
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-08-06 , DOI: 10.1002/slct.201900938 Vinay K Singh 1 , Vineeta Pillai 1 , Shailykumari K. Patel 1 , Lipi Buch 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-08-06 , DOI: 10.1002/slct.201900938 Vinay K Singh 1 , Vineeta Pillai 1 , Shailykumari K. Patel 1 , Lipi Buch 2
Affiliation
|
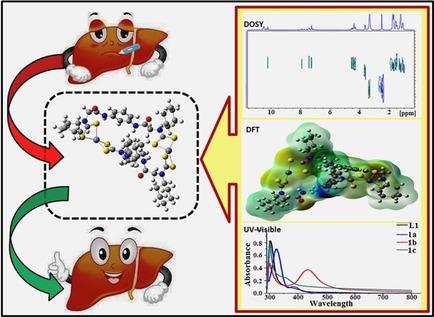
α‐chloroamide 1,3‐bis(2‐chloroacetamido)phenylene (L’) is selected as a lead compound to derive 1,3‐bis(2‐(alkylamino)acetamido)phenylene (L1‐L3). A programmed self‐assembly involving L1‐L3, CS2 and NiII, CuII or ZnII ions affords access to a new series of 32‐membered binuclear macrocyclic dithiocarbamates [MII2‐μ2‐bis‐{(κ2S,S‐S2CN(R)CH2CONH)2C6H4}]{R=Cy, M=NiII 1 a, CuII 1 b, ZnII 1 c; R=iPr, M=NiII 2 a, CuII 2 b, ZnII 2 c; R=nBu, M=NiII 3 a, CuII 3 b, ZnII 3 c}. Compounds were characterized by spectroscopic (1H, 13C, DEPT 135, 1H DOSY NMR, HRMS/ESI MS, UV‐visible, Fluorescence, IR) and by the TGA. Evidently, L’ forms a fascinating 2D infinite supramolecular molecular sheet. All the compounds were screened for their in vitro cytotoxic activity against malignant tumor Hep G2 (hepatoma) cell line by the MTT assay. A majority of compounds ca L’, L1, 1 a, 1 b, 1 c, 2 a, 2 b, 2 c, L3,3 a, 3 b, 3 c display IC50 values lower than cisplatin and specificity for carcinoma Hep G2 over normal liver cell line (WRL‐68). Evidently cytotoxic potentials of L1‐L3 improved tremendously upon formation of their corresponding bimetallic dithiocarbamate complexes. The shrinking of cells can be clearly visualized by acridine orange/ethidium bromide (AO/EB) staining indicating the induction of apoptosis as part of the mechanism of action of these compounds. Further, DFT level calculations have been performed on representative compounds to reinforce the experimental results.
中文翻译:

通过改变双核二硫代氨基甲酸酯络合物中的连接物从二苯醚到二苯甲烷,再到苯,改善细胞毒性:合成和细胞毒性研究
选择α-氯酰胺1,3-双(2-氯乙酰胺基)亚苯基(L')作为衍生化合物来衍生1,3-双(2-(烷基氨基)乙酰胺基)亚苯基(L 1 -L 3)。编程的自组装涉及大号1 -L 3,CS 2和Ni II,铜II或Zn II离子,得到一个新的系列的访问的32元大环双核二硫代氨基甲酸[M II 2 - μ 2 -双- {(κ 2 S,S -S 2 CN(R)CH 2 CONH)2 C 6 H 4}] {R = Cy,M = Ni II 1a,Cu II 1b,Zn II 1c;R =我镨,M =镍II 2,铜II 2b中,锌II 2 C ; R =n Bu, M = Ni II 3a,Cu II 3b,Zn II 3c }。化合物,其特征在于通过光谱(1个H,13 C,DEPT 135 1H DOSY NMR,HRMS / ESI MS,紫外可见光,荧光,IR)和通过TGA。显然,L'形成了一个引人入胜的2D无限超分子分子片。通过MTT测定法筛选所有化合物对恶性肿瘤Hep G2(肝癌)细胞系的体外细胞毒性活性。大多数的化合物CA L”,L 1,1,图1b,图1c,图2a,2b和2c,L 3,图3a,图3b,图3c显示IC 50值比顺铂和特异性降低正常肝细胞系(WRL-68)上的Hep G2癌。L 1到L 3的明显细胞毒性潜能形成相应的双金属二硫代氨基甲酸酯配合物后,性能得到极大改善。通过a啶橙/溴化乙锭(AO / EB)染色可以清楚地看到细胞的萎缩,表明细胞凋亡的诱导是这些化合物作用机制的一部分。此外,已经对代表性化合物进行了DFT含量计算,以增强实验结果。
更新日期:2019-08-06
中文翻译:

通过改变双核二硫代氨基甲酸酯络合物中的连接物从二苯醚到二苯甲烷,再到苯,改善细胞毒性:合成和细胞毒性研究
选择α-氯酰胺1,3-双(2-氯乙酰胺基)亚苯基(L')作为衍生化合物来衍生1,3-双(2-(烷基氨基)乙酰胺基)亚苯基(L 1 -L 3)。编程的自组装涉及大号1 -L 3,CS 2和Ni II,铜II或Zn II离子,得到一个新的系列的访问的32元大环双核二硫代氨基甲酸[M II 2 - μ 2 -双- {(κ 2 S,S -S 2 CN(R)CH 2 CONH)2 C 6 H 4}] {R = Cy,M = Ni II 1a,Cu II 1b,Zn II 1c;R =我镨,M =镍II 2,铜II 2b中,锌II 2 C ; R =n Bu, M = Ni II 3a,Cu II 3b,Zn II 3c }。化合物,其特征在于通过光谱(1个H,13 C,DEPT 135 1H DOSY NMR,HRMS / ESI MS,紫外可见光,荧光,IR)和通过TGA。显然,L'形成了一个引人入胜的2D无限超分子分子片。通过MTT测定法筛选所有化合物对恶性肿瘤Hep G2(肝癌)细胞系的体外细胞毒性活性。大多数的化合物CA L”,L 1,1,图1b,图1c,图2a,2b和2c,L 3,图3a,图3b,图3c显示IC 50值比顺铂和特异性降低正常肝细胞系(WRL-68)上的Hep G2癌。L 1到L 3的明显细胞毒性潜能形成相应的双金属二硫代氨基甲酸酯配合物后,性能得到极大改善。通过a啶橙/溴化乙锭(AO / EB)染色可以清楚地看到细胞的萎缩,表明细胞凋亡的诱导是这些化合物作用机制的一部分。此外,已经对代表性化合物进行了DFT含量计算,以增强实验结果。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号