当前位置:
X-MOL 学术
›
Anal. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A new approach for the separation, characterization and testing of potential prionoid protein aggregates through hollow-fiber flow field-flow fractionation and multi-angle light scattering
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2019-12-01 , DOI: 10.1016/j.aca.2019.08.003 Valentina Marassi 1 , Francesca Beretti 2 , Barbara Roda 1 , Andrea Alessandrini 3 , Paolo Facci 3 , Tullia Maraldi 2 , Andrea Zattoni 1 , Pierluigi Reschiglian 1 , Marinella Portolani 2
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2019-12-01 , DOI: 10.1016/j.aca.2019.08.003 Valentina Marassi 1 , Francesca Beretti 2 , Barbara Roda 1 , Andrea Alessandrini 3 , Paolo Facci 3 , Tullia Maraldi 2 , Andrea Zattoni 1 , Pierluigi Reschiglian 1 , Marinella Portolani 2
Affiliation
|
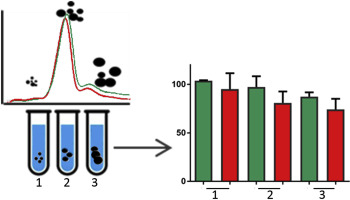
Protein misfolding and aggregation are the common mechanisms in a variety of aggregation-dependent diseases. The compromised proteins often assemble into toxic, accumulating amyloid-like structures of various lengths and their toxicity can also be transferred both in vivo and in vitro a prion-like behavior. The characterization of protein interactions, degradation and conformational dynamics in biological systems still represents an analytical challenge in the prion-like protein comprehension. In our work, we investigated the nature of a transferable cytotoxic agent, presumably a misfolded protein, through the coupling of a multi-detector, non-destructive separation platform based on hollow-fiber flow field-flow fractionation with imaging and downstream in vitro tests. After purification with ion exchange chromatography, the transferable cytotoxic agentwas analyzed with Atomic Force Microscopy and statistical analysis, showing that the concentration of protein dimers and low n-oligomer forms was higher in the cytotoxic sample than in the control preparation. To assess whether the presence of these species was the actual toxic and/or self-propagating factor, we employed HF5 fractionation, with UV and Multi-Angle Light Scattering detection, to define proteins molar mass distribution and abundance, and fractionate the sample into size-homogeneous fractions. These fractions were then tested individually in vitro to investigate the direct correlation with cytotoxicity. Only the later-eluted fraction, which contains high-molar mass aggregates, proved to be toxic onto cell cultures. Moreover, it was observed that the selective transfer of toxicity also occurs for one lower-mass fraction, suggesting that two different mechanisms, acute and later induced toxicity, are in place. These results strongly encourage the efficacy of this platform to enable the identification of protein toxicants.
中文翻译:

一种通过中空纤维流场-流动分级和多角度光散射分离、表征和测试潜在朊蛋白聚集体的新方法
蛋白质错误折叠和聚集是多种聚集依赖性疾病的常见机制。受损的蛋白质通常组装成有毒的、积累的各种长度的淀粉样蛋白结构,它们的毒性也可以在体内和体外转移成朊病毒样行为。生物系统中蛋白质相互作用、降解和构象动力学的表征仍然是朊病毒样蛋白质理解中的分析挑战。在我们的工作中,我们通过基于中空纤维流场流动分级的多检测器、非破坏性分离平台与成像和下游体外测试的耦合,研究了可转移细胞毒性剂的性质,大概是错误折叠的蛋白质. 离子交换层析纯化后,用原子力显微镜和统计分析对可转移的细胞毒剂进行了分析,表明细胞毒样品中的蛋白质二聚体和低n-寡聚体形式的浓度高于对照制剂中的浓度。为了评估这些物质的存在是否是实际的毒性和/或自传播因子,我们采用了 HF5 分馏,结合紫外线和多角度光散射检测,来定义蛋白质的摩尔质量分布和丰度,并将样品分馏成大小-均质部分。然后在体外单独测试这些级分以研究与细胞毒性的直接相关性。只有后来洗脱的部分含有高摩尔质量聚集体,被证明对细胞培养物有毒。而且,据观察,毒性的选择性转移也发生在一个质量较低的部分,这表明存在两种不同的机制,即急性毒性和后期诱导毒性。这些结果有力地促进了该平台在鉴定蛋白质毒物方面的功效。
更新日期:2019-12-01
中文翻译:

一种通过中空纤维流场-流动分级和多角度光散射分离、表征和测试潜在朊蛋白聚集体的新方法
蛋白质错误折叠和聚集是多种聚集依赖性疾病的常见机制。受损的蛋白质通常组装成有毒的、积累的各种长度的淀粉样蛋白结构,它们的毒性也可以在体内和体外转移成朊病毒样行为。生物系统中蛋白质相互作用、降解和构象动力学的表征仍然是朊病毒样蛋白质理解中的分析挑战。在我们的工作中,我们通过基于中空纤维流场流动分级的多检测器、非破坏性分离平台与成像和下游体外测试的耦合,研究了可转移细胞毒性剂的性质,大概是错误折叠的蛋白质. 离子交换层析纯化后,用原子力显微镜和统计分析对可转移的细胞毒剂进行了分析,表明细胞毒样品中的蛋白质二聚体和低n-寡聚体形式的浓度高于对照制剂中的浓度。为了评估这些物质的存在是否是实际的毒性和/或自传播因子,我们采用了 HF5 分馏,结合紫外线和多角度光散射检测,来定义蛋白质的摩尔质量分布和丰度,并将样品分馏成大小-均质部分。然后在体外单独测试这些级分以研究与细胞毒性的直接相关性。只有后来洗脱的部分含有高摩尔质量聚集体,被证明对细胞培养物有毒。而且,据观察,毒性的选择性转移也发生在一个质量较低的部分,这表明存在两种不同的机制,即急性毒性和后期诱导毒性。这些结果有力地促进了该平台在鉴定蛋白质毒物方面的功效。













































 京公网安备 11010802027423号
京公网安备 11010802027423号