当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A single quantum dot-based nanosensor with multilayer of multiple acceptors for ultrasensitive detection of human alkyladenine DNA glycosylase
Chemical Science ( IF 7.6 ) Pub Date : 2019-08-06 , DOI: 10.1039/c9sc02137j Chen-chen Li 1, 2, 3, 4, 5 , Wan-xin Liu 1, 2, 3, 4, 5 , Juan Hu 1, 2, 3, 4, 5 , Chun-yang Zhang 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2019-08-06 , DOI: 10.1039/c9sc02137j Chen-chen Li 1, 2, 3, 4, 5 , Wan-xin Liu 1, 2, 3, 4, 5 , Juan Hu 1, 2, 3, 4, 5 , Chun-yang Zhang 1, 2, 3, 4, 5
Affiliation
|
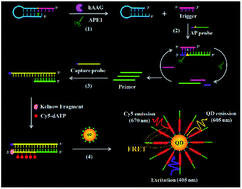
Base excision repair (BER) is an important DNA repair pathway involved in the maintenance of genome stability. As the initiator of BER, DNA glycosylase can remove a damaged base from DNA through cleaving the N-glycosidic bond between the sugar moiety and the damaged base. Accurate quantification of DNA glycosylase is essential for the early diagnosis of various human diseases. However, conventional methods for DNA glycosylase assay usually suffer from poor sensitivity and complex probe design. Herein, we develop a single quantum dot-based nanosensor with multilayer of multiple acceptors for ultrasensitive detection of human alkyladenine DNA glycosylase (hAAG) using apurinic/apyrimidinic endonuclease 1 (APE1)-assisted cyclic cleavage-mediated signal amplification in combination with the DNA polymerase-assisted multiple cyanine 5 (Cy5)-mediated fluorescence resonance energy transfer (FRET). The presence of hAAG induces the cleavage of the hairpin substrate, generating a trigger. The resultant trigger can hybridize with a probe modified with an AP site, initiating the APE1-mediated cyclic cleavage to produce a large number of primers. The primers can subsequently initiate the polymerase-mediated signal amplification with a biotin-modified capture probe as the template, generating the biotin-/multiple Cy5-labeled double-stranded DNAs (dsDNAs). The resultant dsDNAs can assemble onto the QD surface to form the QD-dsDNA-Cy5 nanostructure, leading to efficient FRET from the QD to Cy5 under the excitation of 405 nm. In contrast to the typical QD-based FRET approaches, the assembly of multilayer of multiple Cy5 molecules onto a single QD significantly amplifies the FRET signal. We further verify the FRET model with one donor and multilayered acceptors theoretically and experimentally. This single QD-based nanosensor can sensitively detect hAAG with a detection limit of as low as 4.42 × 10−12 U μL−1. Moreover, it can detect hAAG even in a single cancer cell, and distinguish the cancer cells from the normal cells. Importantly, this single QD-based nanosensor can be used for the kinetic study and inhibition assay, and it may become a universal platform for the detection of other DNA repair enzymes by designing appropriate DNA substrates.
中文翻译:

具有多个受体多层的基于单量子点的纳米传感器,用于超灵敏地检测人烷基腺嘌呤DNA糖基化酶
碱基切除修复(BER)是重要的DNA修复途径,参与基因组稳定性的维持。作为BER的引发剂,DNA糖基化酶可以通过切割糖部分和受损碱基之间的N-糖苷键,从DNA中除去受损碱基。DNA糖基化酶的准确定量对于各种人类疾病的早期诊断至关重要。然而,用于DNA糖基化酶测定的常规方法通常具有灵敏度低和探针设计复杂的缺点。在此处,我们开发了一种基于量子点的纳米传感器,具有多个受体的多层结构,可使用嘌呤/嘧啶内切核酸酶1(APE1)辅助的环状裂解介导的信号放大结合DNA聚合酶辅助的超灵敏检测人烷基腺嘌呤DNA糖基化酶(hAAG)多花青5(Cy5)介导的荧光共振能量转移(FRET)。hAAG的存在会诱导发夹底物的裂解,从而产生触发作用。所得的触发物可以与用AP位点修饰的探针杂交,从而引发APE1介导的环状切割,从而产生大量引物。引物随后可以以生物素修饰的捕获探针为模板来启动聚合酶介导的信号放大,从而生成生物素/多个Cy5标记的双链DNA(dsDNA)。所得的dsDNA可以组装到QD表面上以形成QD-dsDNA-Cy5纳米结构,从而在405 nm的激发下导致从QD到Cy5的有效FRET。与典型的基于QD的FRET方法相比,将多个Cy5分子的多层组装到单个QD上会显着放大FRET信号。我们在理论和实验上进一步验证了具有一个供体和多层受体的FRET模型。这种基于QD的单个纳米传感器可以灵敏地检测hAAG,检测限低至4.42×10 将多个Cy5分子的多层组装到单个QD上会显着放大FRET信号。我们在理论上和实验上进一步验证了具有一个供体和多层受体的FRET模型。这种基于QD的单个纳米传感器可以灵敏地检测hAAG,检测限低至4.42×10 将多个Cy5分子的多层组装到单个QD上会显着放大FRET信号。我们在理论上和实验上进一步验证了具有一个供体和多层受体的FRET模型。这种基于QD的单个纳米传感器可以灵敏地检测hAAG,检测限低至4.42×10-12 UμL -1。此外,它甚至可以在单个癌细胞中检测hAAG,并将癌细胞与正常细胞区分开。重要的是,这种基于QD的纳米传感器可用于动力学研究和抑制测定,并且通过设计适当的DNA底物,它可以成为检测其他DNA修复酶的通用平台。
更新日期:2019-08-12
中文翻译:

具有多个受体多层的基于单量子点的纳米传感器,用于超灵敏地检测人烷基腺嘌呤DNA糖基化酶
碱基切除修复(BER)是重要的DNA修复途径,参与基因组稳定性的维持。作为BER的引发剂,DNA糖基化酶可以通过切割糖部分和受损碱基之间的N-糖苷键,从DNA中除去受损碱基。DNA糖基化酶的准确定量对于各种人类疾病的早期诊断至关重要。然而,用于DNA糖基化酶测定的常规方法通常具有灵敏度低和探针设计复杂的缺点。在此处,我们开发了一种基于量子点的纳米传感器,具有多个受体的多层结构,可使用嘌呤/嘧啶内切核酸酶1(APE1)辅助的环状裂解介导的信号放大结合DNA聚合酶辅助的超灵敏检测人烷基腺嘌呤DNA糖基化酶(hAAG)多花青5(Cy5)介导的荧光共振能量转移(FRET)。hAAG的存在会诱导发夹底物的裂解,从而产生触发作用。所得的触发物可以与用AP位点修饰的探针杂交,从而引发APE1介导的环状切割,从而产生大量引物。引物随后可以以生物素修饰的捕获探针为模板来启动聚合酶介导的信号放大,从而生成生物素/多个Cy5标记的双链DNA(dsDNA)。所得的dsDNA可以组装到QD表面上以形成QD-dsDNA-Cy5纳米结构,从而在405 nm的激发下导致从QD到Cy5的有效FRET。与典型的基于QD的FRET方法相比,将多个Cy5分子的多层组装到单个QD上会显着放大FRET信号。我们在理论和实验上进一步验证了具有一个供体和多层受体的FRET模型。这种基于QD的单个纳米传感器可以灵敏地检测hAAG,检测限低至4.42×10 将多个Cy5分子的多层组装到单个QD上会显着放大FRET信号。我们在理论上和实验上进一步验证了具有一个供体和多层受体的FRET模型。这种基于QD的单个纳米传感器可以灵敏地检测hAAG,检测限低至4.42×10 将多个Cy5分子的多层组装到单个QD上会显着放大FRET信号。我们在理论上和实验上进一步验证了具有一个供体和多层受体的FRET模型。这种基于QD的单个纳米传感器可以灵敏地检测hAAG,检测限低至4.42×10-12 UμL -1。此外,它甚至可以在单个癌细胞中检测hAAG,并将癌细胞与正常细胞区分开。重要的是,这种基于QD的纳米传感器可用于动力学研究和抑制测定,并且通过设计适当的DNA底物,它可以成为检测其他DNA修复酶的通用平台。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号