当前位置:
X-MOL 学术
›
Nat. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomic structures of an entire contractile injection system in both the extended and contracted states.
Nature Microbiology ( IF 20.5 ) Pub Date : 2019-08-05 , DOI: 10.1038/s41564-019-0530-6 Ambroise Desfosses 1, 2 , Hariprasad Venugopal 1, 3 , Tapan Joshi 1 , Jan Felix 2 , Matthew Jessop 1, 2 , Hyengseop Jeong 4 , Jaekyung Hyun 4, 5 , J Bernard Heymann 6 , Mark R H Hurst 7, 8 , Irina Gutsche 2 , Alok K Mitra 1
Nature Microbiology ( IF 20.5 ) Pub Date : 2019-08-05 , DOI: 10.1038/s41564-019-0530-6 Ambroise Desfosses 1, 2 , Hariprasad Venugopal 1, 3 , Tapan Joshi 1 , Jan Felix 2 , Matthew Jessop 1, 2 , Hyengseop Jeong 4 , Jaekyung Hyun 4, 5 , J Bernard Heymann 6 , Mark R H Hurst 7, 8 , Irina Gutsche 2 , Alok K Mitra 1
Affiliation
|
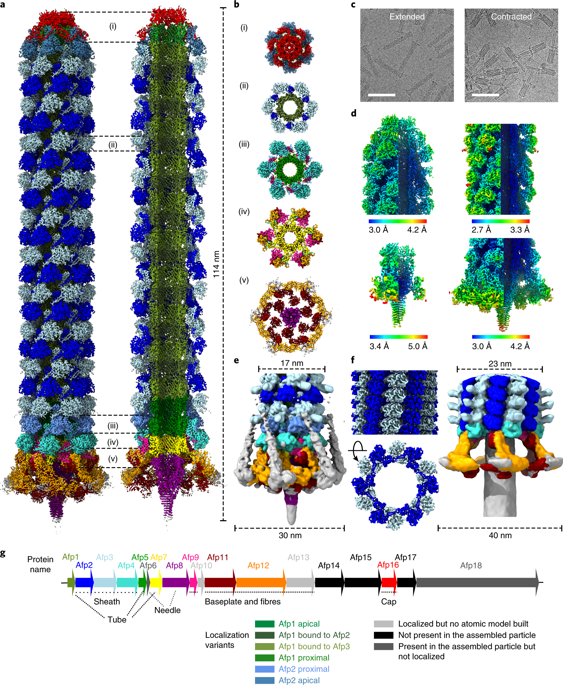
Contractile injection systems are sophisticated multiprotein nanomachines that puncture target cell membranes. Although the number of atomic-resolution insights into contractile bacteriophage tails, bacterial type six secretion systems and R-pyocins is rapidly increasing, structural information on the contraction of bacterial phage-like protein-translocation structures directed towards eukaryotic hosts is scarce. Here, we characterize the antifeeding prophage AFP from Serratia entomophila by cryo-electron microscopy. We present the high-resolution structure of the entire AFP particle in the extended state, trace 11 protein chains de novo from the apical cap to the needle tip, describe localization variants and perform specific structural comparisons with related systems. We analyse inter-subunit interactions and highlight their universal conservation within contractile injection systems while revealing the specificities of AFP. Furthermore, we provide the structure of the AFP sheath-baseplate complex in a contracted state. This study reveals atomic details of interaction networks that accompany and define the contraction mechanism of toxin-delivery tailocins, offering a comprehensive framework for understanding their mode of action and for their possible adaptation as biocontrol agents.
中文翻译:

处于扩张状态和收缩状态的整个收缩注射系统的原子结构。
收缩注射系统是复杂的多蛋白纳米机器,可刺穿靶细胞膜。尽管对可收缩噬菌体尾巴,六型细菌分泌系统和R-球菌素的原子分辨率见解迅速增加,但针对真核宿主的细菌噬菌体样蛋白易位结构收缩的结构信息却很少。在这里,我们通过冷冻电子显微镜表征食虫沙雷氏菌(Serratia entomophila)的反喂食性AFP。我们提出了处于扩展状态的整个AFP颗粒的高分辨率结构,从根尖到针尖的11条蛋白链从头开始,描述了定位变体,并与相关系统进行了特定的结构比较。我们分析了亚基间的相互作用,并强调了它们在收缩注射系统中的普遍保守性,同时揭示了AFP的特异性。此外,我们提供了处于收缩状态的AFP护套-基板复合体的结构。这项研究揭示了相互作用网络的原子细节,这些相互作用网络伴随并定义了毒素传递尾巴素的收缩机制,为理解它们的作用方式以及作为生物防治剂的可能适应提供了一个全面的框架。
更新日期:2019-08-05
中文翻译:

处于扩张状态和收缩状态的整个收缩注射系统的原子结构。
收缩注射系统是复杂的多蛋白纳米机器,可刺穿靶细胞膜。尽管对可收缩噬菌体尾巴,六型细菌分泌系统和R-球菌素的原子分辨率见解迅速增加,但针对真核宿主的细菌噬菌体样蛋白易位结构收缩的结构信息却很少。在这里,我们通过冷冻电子显微镜表征食虫沙雷氏菌(Serratia entomophila)的反喂食性AFP。我们提出了处于扩展状态的整个AFP颗粒的高分辨率结构,从根尖到针尖的11条蛋白链从头开始,描述了定位变体,并与相关系统进行了特定的结构比较。我们分析了亚基间的相互作用,并强调了它们在收缩注射系统中的普遍保守性,同时揭示了AFP的特异性。此外,我们提供了处于收缩状态的AFP护套-基板复合体的结构。这项研究揭示了相互作用网络的原子细节,这些相互作用网络伴随并定义了毒素传递尾巴素的收缩机制,为理解它们的作用方式以及作为生物防治剂的可能适应提供了一个全面的框架。






























 京公网安备 11010802027423号
京公网安备 11010802027423号