当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
3,5–Bis(2–Hydroxyphenyl)–1H–1,2,4–Triazole Derivatives: Synthesis, Crystal Structure and Insecticidal Activity
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-08-05 , DOI: 10.1002/slct.201901706 Chang‐Chun Fan 1 , Shu‐Lin Jiao 1 , Min Qin 1 , Zhi‐Hong Zou 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-08-05 , DOI: 10.1002/slct.201901706 Chang‐Chun Fan 1 , Shu‐Lin Jiao 1 , Min Qin 1 , Zhi‐Hong Zou 1
Affiliation
|
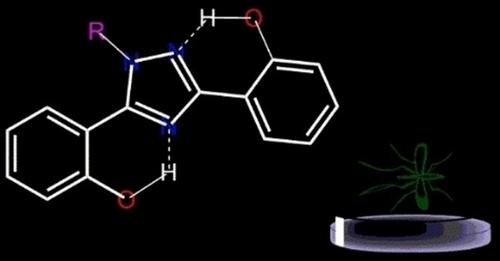
In an effort to discover new molecules with good insecticidal activities, a series of 3,5‐bis (2‐hydroxyphenyl)‐1H‐1,2,4‐triazole derivatives were synthesized. The X–ray crystallographic and Hirshfeld surface calculation study of compound 4 c reveal the structural characteristics and strong intermolecular interactions and also evaluated for it's insecticidal activity. The bioassays data demonstrate that all the target compounds possess remarkable activities against broad bean aphid with the mortalities ranging from 98.12% to 100% at the concentration of 100 mg/L. In particular, methyl 4‐[3,5‐bis(2–hydroxyphenyl)‐1H‐1,2,4‐triazol‐1‐yl] benzoate (85.53%) shows higher activity than dinotefuran (85.25%) at the concentration of 10 mg/L whereas other compounds display low activity at the same concentration. The docking model of compound 4a shows possible mechanism of insecticidal action of target compounds. These results indicate that 3,5−bis (2−hydroxyphenyl) ‐1H‐1,2,4–triazole derivatives could be developed as novel and promising insecticides.
中文翻译:

3,5–双(2-羟基苯基)–1H–1,2,4-三唑衍生物:合成,晶体结构和杀虫活性
为了发现具有良好杀虫活性的新分子,合成了一系列3,5-双(2-羟基苯基)-1H-1,1,2,4-三唑衍生物。化合物4 c的X射线晶体学和Hirshfeld表面计算研究揭示了其结构特征和强大的分子间相互作用,并对其杀虫活性进行了评估。生物测定数据表明,所有目标化合物对蚕豆蚜都有显着活性,在100 mg / L的浓度下,死亡率为98.12%至100%。特别是甲基4- [3,5-双(2-羟基苯基)-1 H苯并[1,2,4-三唑-1]](85.53%)在浓度为10 mg / L时比二甲呋喃(85.25%)更高的活性,而其他化合物在相同浓度下的活性低。化合物4a的对接模型显示了目标化合物杀虫作用的可能机理。这些结果表明,3,5-双(2-羟苯基)-1 H -1,2,4-三唑衍生物可以被开发为新型和有前景的杀虫剂。
更新日期:2019-08-05
中文翻译:

3,5–双(2-羟基苯基)–1H–1,2,4-三唑衍生物:合成,晶体结构和杀虫活性
为了发现具有良好杀虫活性的新分子,合成了一系列3,5-双(2-羟基苯基)-1H-1,1,2,4-三唑衍生物。化合物4 c的X射线晶体学和Hirshfeld表面计算研究揭示了其结构特征和强大的分子间相互作用,并对其杀虫活性进行了评估。生物测定数据表明,所有目标化合物对蚕豆蚜都有显着活性,在100 mg / L的浓度下,死亡率为98.12%至100%。特别是甲基4- [3,5-双(2-羟基苯基)-1 H苯并[1,2,4-三唑-1]](85.53%)在浓度为10 mg / L时比二甲呋喃(85.25%)更高的活性,而其他化合物在相同浓度下的活性低。化合物4a的对接模型显示了目标化合物杀虫作用的可能机理。这些结果表明,3,5-双(2-羟苯基)-1 H -1,2,4-三唑衍生物可以被开发为新型和有前景的杀虫剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号