当前位置:
X-MOL 学术
›
ACS Appl. Polym. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox-Driven Disassembly of Polymer–Chlorambucil Polyprodrug: Delivery of Anticancer Nitrogen Mustard and DNA Alkylation
ACS Applied Polymer Materials ( IF 4.4 ) Pub Date : 2019-08-19 , DOI: 10.1021/acsapm.9b00616 Biswajit Saha , Sudipta Bhattacharyya , Sourav Mete , Arindam Mukherjee , Priyadarsi De
ACS Applied Polymer Materials ( IF 4.4 ) Pub Date : 2019-08-19 , DOI: 10.1021/acsapm.9b00616 Biswajit Saha , Sudipta Bhattacharyya , Sourav Mete , Arindam Mukherjee , Priyadarsi De
|
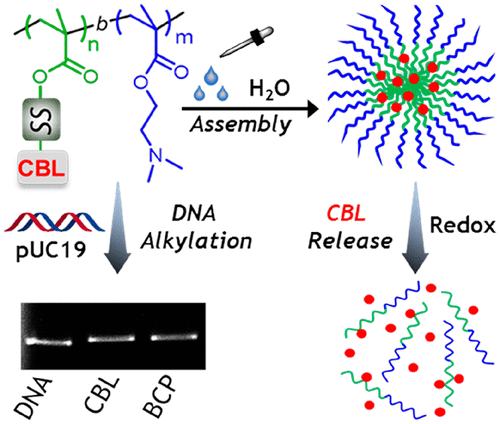
Utilization of nitrogen mustards as anticancer drugs opened the way for precise cancer chemotherapy owing to their ability to cross-link deoxyribonucleic acid (DNA). However, the non-specificity and lower water solubility limit their aptitude to behave as effective anticancer drugs. Herein we embed the clinically approved nitrogen mustard, chlorambucil (CBL), into a redox-responsive polymeric vector to overcome those bottlenecks as well as to achieve better efficacy. We have synthesized amphiphilic diblock copolymers (BCPs) consisting of polymerized segments of a pendent disulfide-labeled CBL prodrug monomer and hydrophilic 2-(dimethylamino)ethyl methacrylate monomer via reversible addition–fragmentation chain transfer polymerization. The well-defined polyprodrug amphiphiles with CBL loading content >40 wt% could form uniform micelles with an average diameter of 45–110 nm through a macromolecular self-assembly strategy. For the release of caged CBL, disassembly of the self-assembled micelles was attained under reducing conditions, confirmed through dynamic light scattering and field emission scanning electron microscopy. In vitro evaluation of CBL release showed that the percentage of drug release was profoundly enhanced up to >90% in the presence of 5 mM d,l-dithiothreitol. Cytotoxicity studies against a triple-negative breast cancer (MDA-MB-231) cell line revealed that the BCP has much better cytotoxicity than the free CBL. Furthermore, the alkylating activity of BCP was confirmed using a colorimetric indicator, 4-(4-nitrobenzyl)pyridine, and upon agarose gel electrophoresis with pUC19 plasmid DNA. Combining the features of redox-responsive drug release and DNA alkylation, BCPs provide insight into drug delivery and disrupt the thiol-rich environment of cancer cells.
中文翻译:

氧化还原驱动的聚合物-苯丁酸氮芥前药的分解:抗癌氮芥末和DNA烷基化的传递。
由于氮芥子能交联脱氧核糖核酸(DNA),因此将其用作抗癌药物为精确的癌症化疗开辟了道路。然而,非特异性和较低的水溶性限制了它们作为有效抗癌药的能力。本文中,我们将经过临床批准的氮芥芥,苯丁酸氮芥(CBL)嵌入到氧化还原反应性聚合物载体中,以克服这些瓶颈并获得更好的功效。我们已经通过可逆的加成-断裂链转移聚合反应合成了两亲性的二嵌段共聚物(BCP),该二嵌段共聚物由悬垂的二硫键标记的CBL前药单体和亲水的甲基丙烯酸2-(二甲基氨基)乙酯单体的聚合链段组成。具有明确定义的多药前两亲物,其CBL负载量> 通过大分子自组装策略,40 wt%可以形成平均直径为45–110 nm的均匀胶束。为了释放笼状的CBL,在还原条件下实现了自组装胶束的拆卸,这通过动态光散射和场发射扫描电子显微镜得以证实。CBL释放的体外评估表明,在5 mM d,1-二硫苏糖醇存在下,药物释放的百分比显着提高至> 90%。针对三阴性乳腺癌(MDA-MB-231)细胞系的细胞毒性研究表明,BCP的细胞毒性比游离CBL更好。此外,使用比色指示剂4-(4-硝基苄基)吡啶,并用pUC19质粒DNA进行琼脂糖凝胶电泳,证实了BCP的烷基化活性。结合氧化还原反应性药物释放和DNA烷基化的特征,BCP提供了对药物输送的洞察力,并破坏了癌细胞中富含硫醇的环境。
更新日期:2019-08-20
中文翻译:

氧化还原驱动的聚合物-苯丁酸氮芥前药的分解:抗癌氮芥末和DNA烷基化的传递。
由于氮芥子能交联脱氧核糖核酸(DNA),因此将其用作抗癌药物为精确的癌症化疗开辟了道路。然而,非特异性和较低的水溶性限制了它们作为有效抗癌药的能力。本文中,我们将经过临床批准的氮芥芥,苯丁酸氮芥(CBL)嵌入到氧化还原反应性聚合物载体中,以克服这些瓶颈并获得更好的功效。我们已经通过可逆的加成-断裂链转移聚合反应合成了两亲性的二嵌段共聚物(BCP),该二嵌段共聚物由悬垂的二硫键标记的CBL前药单体和亲水的甲基丙烯酸2-(二甲基氨基)乙酯单体的聚合链段组成。具有明确定义的多药前两亲物,其CBL负载量> 通过大分子自组装策略,40 wt%可以形成平均直径为45–110 nm的均匀胶束。为了释放笼状的CBL,在还原条件下实现了自组装胶束的拆卸,这通过动态光散射和场发射扫描电子显微镜得以证实。CBL释放的体外评估表明,在5 mM d,1-二硫苏糖醇存在下,药物释放的百分比显着提高至> 90%。针对三阴性乳腺癌(MDA-MB-231)细胞系的细胞毒性研究表明,BCP的细胞毒性比游离CBL更好。此外,使用比色指示剂4-(4-硝基苄基)吡啶,并用pUC19质粒DNA进行琼脂糖凝胶电泳,证实了BCP的烷基化活性。结合氧化还原反应性药物释放和DNA烷基化的特征,BCP提供了对药物输送的洞察力,并破坏了癌细胞中富含硫醇的环境。












































 京公网安备 11010802027423号
京公网安备 11010802027423号