当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Energetics of Doxorubicin Pumping by Human P-Glycoprotein.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2019-08-16 , DOI: 10.1021/acs.jcim.9b00429 Lijie Wang 1 , Lin Zhang 1 , Fufeng Liu 1, 2 , Yan Sun 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2019-08-16 , DOI: 10.1021/acs.jcim.9b00429 Lijie Wang 1 , Lin Zhang 1 , Fufeng Liu 1, 2 , Yan Sun 1
Affiliation
|
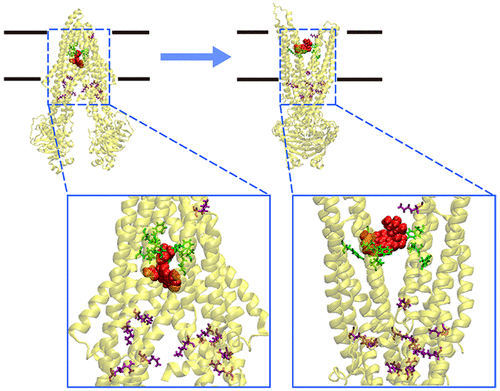
The pumping of antitumor drugs by P-glycoprotein (P-gp) causes multidrug resistance (MDR) and consequent failure of chemotherapy. However, the understanding on the molecular mechanism of P-gp for transporting substrates is still far from adequate. Herein, the transport of a typical antitumor drug, doxorubicin, by P-gp is investigated using targeted molecular dynamics (MD) simulations and molecular mechanics Poisson-Boltzmann surface area (MM-PBSA) analysis. The MM-PBSA analysis identifies the driving forces for the transport of doxorubicin toward the extracellular space as electrostatic repulsions in the initial stage, which are contributed by positively charged residues (R148, K181, K189, K285, K291, K734, R789, K826, K934, and K1000) and then hydrophobic interactions provided by hydrophobic residues (L65, M69, F336, I340, F343, Y953, V982, F983, and M986). The contributions of these residues are further validated by targeted MD simulations, which shows blocked pumping after the mutation of these important residues to glycine. The MM-PBSA and minimum distance analyses of each residue during the transport reveal that the positively charged residues promote the transport of doxorubicin through long-range electrostatic repulsions and the hydrophobic residues provide a pathway through continuous hydrophobic interactions to maintain the transport. The results have thus provided molecular insights into the function of P-gp and would be beneficial in the design of potent P-gp inhibitors against MDR in the medication of cancers.
中文翻译:

人P-糖蛋白对阿霉素泵送的分子能量学。
P-糖蛋白(P-gp)泵送抗肿瘤药物会引起多药耐药性(MDR),并因此导致化疗失败。但是,对P-gp传输底物的分子机理的理解仍然远远不够。本文中,使用靶向分子动力学(MD)模拟和分子力学泊松-玻尔兹曼表面积(MM-PBSA)分析研究了P-gp对典型抗肿瘤药物阿霉素的转运。MM-PBSA分析确定了在初始阶段将阿霉素向细胞外空间迁移的驱动力是静电排斥,这是由带正电荷的残基(R148,K181,K189,K285,K291,K734,R789,K826, K934和K1000),然后由疏水残基(L65,M69,F336,I340,F343,Y953,V982,F983,和M986)。这些残基的贡献通过有针对性的MD模拟得到了进一步验证,该模拟表明这些重要残基突变为甘氨酸后泵浦受阻。MM-PBSA和转运过程中每个残基的最小距离分析表明,带正电荷的残基通过长距离静电排斥促进阿霉素的转运,而疏水残基则提供了通过持续疏水相互作用维持转运的途径。因此,该结果为P-gp的功能提供了分子方面的见识,并且在设计抗癌药物中针对MDR的有效P-gp抑制剂时将是有益的。这些表明这些重要残基突变为甘氨酸后,泵浦受阻。MM-PBSA和转运过程中每个残基的最小距离分析表明,带正电荷的残基通过长距离静电排斥促进阿霉素的转运,而疏水残基则提供了通过持续疏水相互作用维持转运的途径。因此,该结果为P-gp的功能提供了分子方面的见识,并且在设计抗癌药物中针对MDR的有效P-gp抑制剂时将是有益的。这些表明这些重要残基突变为甘氨酸后,泵浦受阻。MM-PBSA和转运过程中每个残基的最小距离分析表明,带正电荷的残基通过长距离静电排斥促进阿霉素的转运,而疏水残基则提供了通过持续疏水相互作用维持转运的途径。因此,该结果为P-gp的功能提供了分子方面的见识,并且在设计抗癌药物中针对MDR的有效P-gp抑制剂时将是有益的。MM-PBSA和转运过程中每个残基的最小距离分析表明,带正电荷的残基通过长距离静电排斥促进阿霉素的转运,而疏水残基则提供了通过持续疏水相互作用维持转运的途径。因此,该结果为P-gp的功能提供了分子方面的见识,并且在设计抗癌药物中针对MDR的有效P-gp抑制剂时将是有益的。MM-PBSA和转运过程中每个残基的最小距离分析表明,带正电荷的残基通过长距离静电排斥促进阿霉素的转运,而疏水残基则提供了通过持续疏水相互作用维持转运的途径。因此,该结果为P-gp的功能提供了分子方面的见识,并且在设计抗癌药物中针对MDR的有效P-gp抑制剂时将是有益的。
更新日期:2019-08-18
中文翻译:

人P-糖蛋白对阿霉素泵送的分子能量学。
P-糖蛋白(P-gp)泵送抗肿瘤药物会引起多药耐药性(MDR),并因此导致化疗失败。但是,对P-gp传输底物的分子机理的理解仍然远远不够。本文中,使用靶向分子动力学(MD)模拟和分子力学泊松-玻尔兹曼表面积(MM-PBSA)分析研究了P-gp对典型抗肿瘤药物阿霉素的转运。MM-PBSA分析确定了在初始阶段将阿霉素向细胞外空间迁移的驱动力是静电排斥,这是由带正电荷的残基(R148,K181,K189,K285,K291,K734,R789,K826, K934和K1000),然后由疏水残基(L65,M69,F336,I340,F343,Y953,V982,F983,和M986)。这些残基的贡献通过有针对性的MD模拟得到了进一步验证,该模拟表明这些重要残基突变为甘氨酸后泵浦受阻。MM-PBSA和转运过程中每个残基的最小距离分析表明,带正电荷的残基通过长距离静电排斥促进阿霉素的转运,而疏水残基则提供了通过持续疏水相互作用维持转运的途径。因此,该结果为P-gp的功能提供了分子方面的见识,并且在设计抗癌药物中针对MDR的有效P-gp抑制剂时将是有益的。这些表明这些重要残基突变为甘氨酸后,泵浦受阻。MM-PBSA和转运过程中每个残基的最小距离分析表明,带正电荷的残基通过长距离静电排斥促进阿霉素的转运,而疏水残基则提供了通过持续疏水相互作用维持转运的途径。因此,该结果为P-gp的功能提供了分子方面的见识,并且在设计抗癌药物中针对MDR的有效P-gp抑制剂时将是有益的。这些表明这些重要残基突变为甘氨酸后,泵浦受阻。MM-PBSA和转运过程中每个残基的最小距离分析表明,带正电荷的残基通过长距离静电排斥促进阿霉素的转运,而疏水残基则提供了通过持续疏水相互作用维持转运的途径。因此,该结果为P-gp的功能提供了分子方面的见识,并且在设计抗癌药物中针对MDR的有效P-gp抑制剂时将是有益的。MM-PBSA和转运过程中每个残基的最小距离分析表明,带正电荷的残基通过长距离静电排斥促进阿霉素的转运,而疏水残基则提供了通过持续疏水相互作用维持转运的途径。因此,该结果为P-gp的功能提供了分子方面的见识,并且在设计抗癌药物中针对MDR的有效P-gp抑制剂时将是有益的。MM-PBSA和转运过程中每个残基的最小距离分析表明,带正电荷的残基通过长距离静电排斥促进阿霉素的转运,而疏水残基则提供了通过持续疏水相互作用维持转运的途径。因此,该结果为P-gp的功能提供了分子方面的见识,并且在设计抗癌药物中针对MDR的有效P-gp抑制剂时将是有益的。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号