当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a lipoplex-type mRNA carrier composed of an ionizable lipid with a vitamin E scaffold and the KALA peptide for use as an ex vivo dendritic cell-based cancer vaccine.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-08-03 , DOI: 10.1016/j.jconrel.2019.08.002 Naho Tateshita 1 , Naoya Miura 1 , Hiroki Tanaka 1 , Takeshi Masuda 2 , Sumio Ohtsuki 2 , Kota Tange 3 , Yuta Nakai 3 , Hiroki Yoshioka 3 , Hidetaka Akita 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-08-03 , DOI: 10.1016/j.jconrel.2019.08.002 Naho Tateshita 1 , Naoya Miura 1 , Hiroki Tanaka 1 , Takeshi Masuda 2 , Sumio Ohtsuki 2 , Kota Tange 3 , Yuta Nakai 3 , Hiroki Yoshioka 3 , Hidetaka Akita 1
Affiliation
|
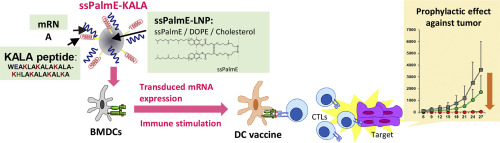
A dendritic cells (DCs)-based vaccine (DC-vaccine) system is an attractive technology for eliciting antigen-specific immune responses that can protect subjects from infectious diseases and for curing various types of cancers. For the insertion of a foreign antigen to DCs, the transfection of an antigen-coding mRNA to the cells is a promising approach. In order to introduce an antigen, a carrier for mRNA transfection is required, since the mRNA molecule per se is unstable in serum-containing medium. We previously reported on an ionizable lipid-like material with vitamin E-scaffolds (ssPalmE) as a material for a lipid nanoparticle (LNP)-based carrier for nucleic acids. In the present study, we report on the development of a lipoplex-type mRNA carrier for use as a DC-vaccine by using a combination of an ssPalmE-LNP and an α-helical cationic peptide "KALA" (ssPalmE-KALA). The transfection of mRNAs complexed with the ssPalmE-KALA achieved a significantly higher protein expression and the production of proinflammatory cytokines from murine bone marrow derived DCs (BMDCs) in comparison with a lipoplex that was prepared with an ssPalm with fatty acid-scaffolds (myristic acid; ssPalmM-KALA). A cellular uptake process and a pH-responsive membrane-destabilization activity cannot explain the preferred protein expression and immune-stimulation caused by the ssPalmE-KALA. Proteomic analyses suggest that transfection with the ssPalmM-KALA stimulates a down-regulatory pathway of translation, while the transfection with the ssPalmE-KALA does not stimulate it. In the vaccination with the BMDCs that were preliminarily transfected with an ovalbumin (OVA)-encoding mRNA elicited the induction OVA specific cytotoxic T-lymphocyte activity in vivo. In parallel, the vaccination induced significant prophylactic anti-tumor effects against a model tumor that stably expressed the OVA protein. Based on the above findings, the ssPalmE-KALA appears to be a potent ex vivo DCs-based RNA vaccine platform.
中文翻译:

由可电离的脂质与维生素E支架和KALA肽组成的脂复合物型mRNA载体的开发,可用作离体树突状细胞癌疫苗。
基于树突细胞(DCs)的疫苗(DC-vaccine)系统是一种引人注目的技术,可引起抗原特异性免疫反应,从而保护受试者免受传染病的侵害并治愈各种类型的癌症。为了将外源抗原插入DC,将编码抗原的mRNA转染至细胞是有前途的方法。为了导入抗原,需要mRNA转染的载体,因为mRNA分子本身在含血清的培养基中不稳定。我们之前曾报道过一种可离子化的类脂质材料,其中维生素E支架(ssPalmE)作为基于脂质纳米颗粒(LNP)的核酸载体的材料。在目前的研究中,我们报道了通过使用ssPalmE-LNP和α-螺旋阳离子肽“ KALA”(ssPalmE-KALA)的组合,用于DC疫苗的脂质复合物型mRNA载体的开发。与用ssPalm和脂肪酸支架(肉豆蔻酸)制备的脂质复合物相比,与ssPalmE-KALA复合的mRNA的转染获得了明显更高的蛋白质表达,并从鼠骨髓来源的DCs(BMDCs)产生了促炎性细胞因子。 ; ssPalmM-KALA)。细胞的吸收过程和pH响应性的膜去稳定活性不能解释ssPalmE-KALA引起的优选蛋白表达和免疫刺激。蛋白质组学分析表明,用ssPalmM-KALA转染可刺激翻译的下调途径,而用ssPalmE-KALA转染不会刺激它。在用预先用卵清蛋白(OVA)编码的mRNA转染的BMDC进行的疫苗接种中,体内诱导了OVA特异性细胞毒性T淋巴细胞的诱导。同时,疫苗接种对稳定表达OVA蛋白的模型肿瘤具有明显的预防性抗肿瘤作用。基于以上发现,ssPalmE-KALA似乎是一种有效的基于离体DC的RNA疫苗平台。
更新日期:2019-08-03
中文翻译:

由可电离的脂质与维生素E支架和KALA肽组成的脂复合物型mRNA载体的开发,可用作离体树突状细胞癌疫苗。
基于树突细胞(DCs)的疫苗(DC-vaccine)系统是一种引人注目的技术,可引起抗原特异性免疫反应,从而保护受试者免受传染病的侵害并治愈各种类型的癌症。为了将外源抗原插入DC,将编码抗原的mRNA转染至细胞是有前途的方法。为了导入抗原,需要mRNA转染的载体,因为mRNA分子本身在含血清的培养基中不稳定。我们之前曾报道过一种可离子化的类脂质材料,其中维生素E支架(ssPalmE)作为基于脂质纳米颗粒(LNP)的核酸载体的材料。在目前的研究中,我们报道了通过使用ssPalmE-LNP和α-螺旋阳离子肽“ KALA”(ssPalmE-KALA)的组合,用于DC疫苗的脂质复合物型mRNA载体的开发。与用ssPalm和脂肪酸支架(肉豆蔻酸)制备的脂质复合物相比,与ssPalmE-KALA复合的mRNA的转染获得了明显更高的蛋白质表达,并从鼠骨髓来源的DCs(BMDCs)产生了促炎性细胞因子。 ; ssPalmM-KALA)。细胞的吸收过程和pH响应性的膜去稳定活性不能解释ssPalmE-KALA引起的优选蛋白表达和免疫刺激。蛋白质组学分析表明,用ssPalmM-KALA转染可刺激翻译的下调途径,而用ssPalmE-KALA转染不会刺激它。在用预先用卵清蛋白(OVA)编码的mRNA转染的BMDC进行的疫苗接种中,体内诱导了OVA特异性细胞毒性T淋巴细胞的诱导。同时,疫苗接种对稳定表达OVA蛋白的模型肿瘤具有明显的预防性抗肿瘤作用。基于以上发现,ssPalmE-KALA似乎是一种有效的基于离体DC的RNA疫苗平台。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号