International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2019-08-02 , DOI: 10.1016/j.ijhydene.2019.07.040 Ahmed Tijani F. Afolabi , Chun-Zhu Li , Panagiotis N. Kechagiopoulos
|
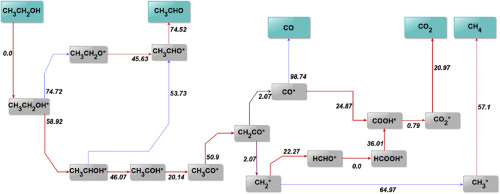
Hydrogen production via the steam reforming of biomass-derived ethanol is a promising environmental alternative to the use of fossil fuels and a means of clean power generation. A microkinetic modelling study of ethanol steam reforming (ESR) on Nickel is presented for the first time and validated with minimal parameter fitting against experimental data collected over a Ni/SiO2 catalyst. The thermodynamically consistent model utilises Transition State Theory and the UBI-QEP method for the determination of kinetic parameters and is able to describe correctly experimental trends across a wide range of conditions. The kinetically controlling reaction steps are predicted to occur in the dehydrogenation pathway of ethanol, with the latter found to proceed primarily via the formation of 1-hydroxyethyl. C-C bond cleavage is predicted to take place at the ketene intermediate leading to the formation of CH2 and CO surface species. The latter intermediates proceed to react according to methane steam reforming and water-gas shift pathways that are enhanced by the presence of water derived OH species. The experimentally observed negative reaction order for water is explained by the model predictions via surface saturation effects of adsorbed water species. The model results highlight a possible distinction between ethanol decomposition pathways as predicted by DFT calculations on Ni close-packed surfaces and ethanol steam reforming pathways at the broad range of experimental conditions considered.
中文翻译:

Ni / SiO 2上乙醇水蒸气重整的动力学模型及反应路径分析
通过生物质衍生的乙醇的蒸汽重整制氢是使用化石燃料和清洁发电手段的一种有前途的环境替代方案。首次提出了镍上乙醇蒸汽重整(ESR)的微动力学模型研究,并针对与在Ni / SiO 2上收集的实验数据进行的最小参数拟合验证了该模型的有效性。催化剂。热力学一致的模型利用过渡状态理论和UBI-QEP方法确定动力学参数,并能够正确描述各种条件下的实验趋势。动力学控制反应步骤预计在乙醇的脱氢途径中发生,发现后者主要通过1-羟乙基的形成进行。预计CC键裂解发生在乙烯酮中间体上,导致CH 2的形成和一氧化碳表面物种。后者的中间体根据甲烷蒸汽重整和水煤气转换路径进行反应,这些路径因水衍生的OH种类的存在而增强。通过模型预测,通过吸附水物种的表面饱和效应,可以解释实验观察到的水的负反应阶数。模型结果突显了在密闭的Ni表面上通过DFT计算所预测的乙醇分解途径与乙醇蒸汽重整途径之间可能存在的区别,该广泛的实验条件已考虑到这一点。































 京公网安备 11010802027423号
京公网安备 11010802027423号