当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Second-order isothermal reaction in a semi-batch reactor: modeling, exact analytical solution, and experimental verification
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2019-08-01 , DOI: 10.1039/c9re00174c Mana Kord 1, 2, 3, 4 , Ali Nematollahzadeh 1, 2, 3, 4 , Behruz Mirzayi 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2019-08-01 , DOI: 10.1039/c9re00174c Mana Kord 1, 2, 3, 4 , Ali Nematollahzadeh 1, 2, 3, 4 , Behruz Mirzayi 1, 2, 3, 4
Affiliation
|
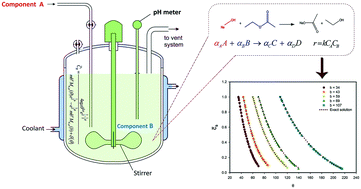
Mathematical modeling of semi-batch reactors (SBRs) can help to control the reaction conditions and product properties through tuning the concentration or flow rate of the external feed of reactants. In the present work, an isothermal SBR behavior with irreversible bimolecular type second-order reactions was investigated both mathematically and experimentally. The governing non-linear differential equation was solved analytically using hypergeometric Whittaker functions. The obtained analytical results were compared with numerical and approximate solutions to verify the correctness and accuracy of the exact solution. In order to determine the validity of the mathematical model, a set of experiments were performed in the reactor and the results were compared with the mathematical solution over a wide range of operating conditions by varying the model parameters. It was observed that the analytical solutions are in good agreement with the experimental data. The relative error between the experimental data and the exact analytical solution in the calculation of the reactant concentration inside the reactor was 0.1–0.3% and that for the feed solution was in the range of 0.7–15%. The obtained exact solution can be beneficial to chemical reactor design and control.
中文翻译:

半间歇式反应器中的二阶等温反应:建模,精确的分析溶液和实验验证
半间歇式反应器(SBR)的数学模型可通过调整反应物外部进料的浓度或流速来帮助控制反应条件和产物性质。在本工作中,通过数学和实验研究了具有不可逆双分子型二级反应的等温SBR行为。使用超几何Whittaker函数解析地求解了控制非线性微分方程。将获得的分析结果与数值解和近似解进行比较,以验证精确解的正确性和准确性。为了确定数学模型的有效性,在反应堆中进行了一系列实验,并通过改变模型参数,将结果与各种工作条件下的数学解进行了比较。据观察,分析溶液与实验数据非常吻合。计算反应器内反应物浓度时,实验数据与精确分析溶液之间的相对误差为0.1–0.3%,进料溶液的相对误差为0.7–15%。所获得的精确解决方案可有利于化学反应器的设计和控制。1-0.3%,进料溶液的浓度在0.7-15%的范围内。所获得的精确解决方案可有利于化学反应器的设计和控制。1-0.3%,进料溶液的浓度在0.7-15%的范围内。所获得的精确解决方案可有利于化学反应器的设计和控制。
更新日期:2019-10-23
中文翻译:

半间歇式反应器中的二阶等温反应:建模,精确的分析溶液和实验验证
半间歇式反应器(SBR)的数学模型可通过调整反应物外部进料的浓度或流速来帮助控制反应条件和产物性质。在本工作中,通过数学和实验研究了具有不可逆双分子型二级反应的等温SBR行为。使用超几何Whittaker函数解析地求解了控制非线性微分方程。将获得的分析结果与数值解和近似解进行比较,以验证精确解的正确性和准确性。为了确定数学模型的有效性,在反应堆中进行了一系列实验,并通过改变模型参数,将结果与各种工作条件下的数学解进行了比较。据观察,分析溶液与实验数据非常吻合。计算反应器内反应物浓度时,实验数据与精确分析溶液之间的相对误差为0.1–0.3%,进料溶液的相对误差为0.7–15%。所获得的精确解决方案可有利于化学反应器的设计和控制。1-0.3%,进料溶液的浓度在0.7-15%的范围内。所获得的精确解决方案可有利于化学反应器的设计和控制。1-0.3%,进料溶液的浓度在0.7-15%的范围内。所获得的精确解决方案可有利于化学反应器的设计和控制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号