Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-07-31 , DOI: 10.1016/j.bmc.2019.07.051 Yifan Feng 1 , Weiming Duan 1 , Shu Fan 1 , Hao Zhang 1 , San-Qi Zhang 1 , Minhang Xin 1
|
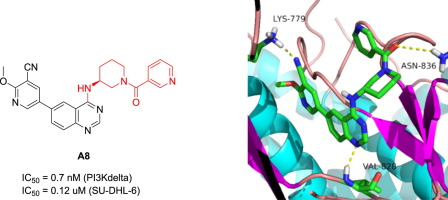
PI3Kδ is an intriguing target for developing anti-cancer agent. In this study, a new series of 4-(piperid-3-yl)amino substituted 6-pyridylquinazoline derivatives were synthesized. After biological evaluation, compounds A5 and A8 were identified as potent PI3Kδ inhibitors, with IC50 values of 1.3 and 0.7 nM, respectively, which are equivalent to or better than idelalisib (IC50 = 1.2 nM). Further PI3K isoforms selectivity evaluation showed that compound A5 afforded excellent PI3Kδ selectivity over PI3Kα, PI3Kβ and PI3Kγ. A8 exhibited superior PI3Kδ/γ selectivity over PI3Kα and PI3Kβ. Moreover, compounds A5 and A8 selectively exhibited anti-proliferation against SU-DHL-6 in vitro with IC50 values of 0.16 and 0.12 μM. Western blot analysis indicated that A8 could attenuate the AKTS473 phosphorylation. Molecular docking study suggested that A8 formed three key H-bonds action with PI3Kδ, which may account for its potent inhibition of PI3Kδ. These findings indicate that 4-(piperid-3-yl)amino substituted 6-pyridylquinazoline derivatives were potent PI3Kδ inhibitors with distinctive PI3K-isoforms and anti-proliferation profiles.
中文翻译:

4-(哌啶-3-基)氨基取代的6-吡啶基喹唑啉类化合物作为强效PI3Kδ抑制剂的合成及生物学评估。
PI3Kδ是开发抗癌药物的引人入胜的目标。在这项研究中,合成了一系列新的4-(哌啶-3-基)氨基取代的6-吡啶基喹唑啉衍生物。经过生物学评估后,化合物A5和A8被确定为有效的PI3Kδ抑制剂,IC 50值分别为1.3和0.7 nM,与艾屈拉西布相当或更好(IC 50 = 1.2 nM)。进一步的PI3K同工型选择性评估表明,化合物A5相对于PI3Kα,PI3Kβ和PI3Kγ具有优异的PI3Kδ选择性。A8表现出优于PI3Kα和PI3Kβ的PI3Kδ/γ选择性。此外,化合物A5和A8选择性地表现出对SU-DHL-6的体外抗增殖,IC 50值为0.16和0.12μM。Western印迹分析表明,A8可以减弱AKT S473的磷酸化。分子对接研究表明,A8与PI3Kδ形成了三个关键的H键作用,这可能解释了其对PI3Kδ的有效抑制作用。这些发现表明4-(哌啶-3-基)氨基取代的6-吡啶基喹唑啉衍生物是有效的PI3Kδ抑制剂,具有独特的PI3K同工型和抗增殖特性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号