当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Repurposing the Pummerer Rearrangement: Determination of Methionine Sulfoxides in Peptides.
ChemBioChem ( IF 2.6 ) Pub Date : 2019-10-25 , DOI: 10.1002/cbic.201900463 Carolyn C Woodroofe 1 , Joseph Ivanic 2 , Sarah Monti 3 , Rodney L Levine 3 , Rolf E Swenson 1
ChemBioChem ( IF 2.6 ) Pub Date : 2019-10-25 , DOI: 10.1002/cbic.201900463 Carolyn C Woodroofe 1 , Joseph Ivanic 2 , Sarah Monti 3 , Rodney L Levine 3 , Rolf E Swenson 1
Affiliation
|
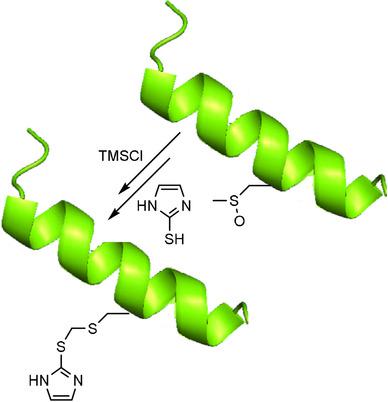
The reversible oxidation of methionine residues in proteins has emerged as a biologically important post-translational modification. However, detection and quantitation of methionine sulfoxide in proteins is difficult. Our aim is to develop a method for specifically derivatizing methionine sulfoxide residues. We report a Pummerer rearrangement of methionine sulfoxide treated sequentially with trimethylsilyl chloride and then 2-mercaptoimidazole or pyridine-2-thiol to produce a dithioacetal product. This derivative is stable to standard mass spectrometry conditions, and its formation identified oxidized methionine residues. The scope and requirements of dithioacetal formation are reported for methionine sulfoxide and model substrates. The reaction intermediates have been investigated by computational techniques and by 13 C NMR spectroscopy. These provide evidence for an α-chlorinated intermediate. The derivatization allows for detection and quantitation of methionine sulfoxide in proteins by mass spectrometry and potentially by immunochemical methods.
中文翻译:

重新利用 Pummerer 重排:测定肽中的甲硫氨酸亚砜。
蛋白质中蛋氨酸残基的可逆氧化已成为生物学上重要的翻译后修饰。然而,蛋白质中甲硫氨酸亚砜的检测和定量是困难的。我们的目标是开发一种专门衍生甲硫氨酸亚砜残基的方法。我们报告了用三甲基氯硅烷依次处理的甲硫氨酸亚砜的 Pummerer 重排,然后是 2-巯基咪唑或吡啶-2-硫醇,以产生二硫缩醛产物。该衍生物在标准质谱条件下是稳定的,并且其形成鉴定了氧化的蛋氨酸残基。报告了甲硫氨酸亚砜和模型底物的二硫缩醛形成的范围和要求。反应中间体已通过计算技术和 13 C NMR 光谱学进行了研究。这些为α-氯化中间体提供了证据。衍生化允许通过质谱和可能通过免疫化学方法检测和定量蛋白质中的甲硫氨酸亚砜。
更新日期:2019-10-25
中文翻译:

重新利用 Pummerer 重排:测定肽中的甲硫氨酸亚砜。
蛋白质中蛋氨酸残基的可逆氧化已成为生物学上重要的翻译后修饰。然而,蛋白质中甲硫氨酸亚砜的检测和定量是困难的。我们的目标是开发一种专门衍生甲硫氨酸亚砜残基的方法。我们报告了用三甲基氯硅烷依次处理的甲硫氨酸亚砜的 Pummerer 重排,然后是 2-巯基咪唑或吡啶-2-硫醇,以产生二硫缩醛产物。该衍生物在标准质谱条件下是稳定的,并且其形成鉴定了氧化的蛋氨酸残基。报告了甲硫氨酸亚砜和模型底物的二硫缩醛形成的范围和要求。反应中间体已通过计算技术和 13 C NMR 光谱学进行了研究。这些为α-氯化中间体提供了证据。衍生化允许通过质谱和可能通过免疫化学方法检测和定量蛋白质中的甲硫氨酸亚砜。















































 京公网安备 11010802027423号
京公网安备 11010802027423号