当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Imatinib by C–N Coupling Reaction of Primary Amide and Bromo-Substituted Pyrimidine Amine
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-07-31 00:00:00 , DOI: 10.1021/acs.oprd.9b00227 Cuiling Wang 1 , Xiao Bai 1 , Rui Wang 1 , Xudong Zheng 1 , Xiumei Ma 1 , Huan Chen 1 , Yun Ai 1, 2 , Yajun Bai 3 , Yifeng Liu 4
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-07-31 00:00:00 , DOI: 10.1021/acs.oprd.9b00227 Cuiling Wang 1 , Xiao Bai 1 , Rui Wang 1 , Xudong Zheng 1 , Xiumei Ma 1 , Huan Chen 1 , Yun Ai 1, 2 , Yajun Bai 3 , Yifeng Liu 4
Affiliation
|
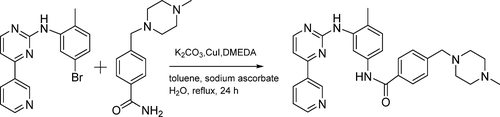
A new method for imatinib synthesis is described by using the C–N coupling reaction of 4-(4-methylpiperazine-1-methyl)benzamide with N-(5-bromo-2-tolyl)-4-(3-pyridyl)pyrimidin-2-amine to form imatinib. In this synthetic route, the high efficiency and high selectivity of nano-ZnO as a catalyst is key to the mild hydrolysis of 4-(4-methylpiperazine-1-methyl)benzonitrile into the corresponding amide. The total imatinib yield was 51.3%, and the purity was 99.9%. This simple and effective synthetic pathway avoids gene-impurity production (as classified by the FDA Center for Drug Evaluation and Research), and the synthesis is environmentally friendly with a short reaction time.
中文翻译:

伯酰胺与溴取代的嘧啶胺的CN偶联反应合成伊马替尼
通过使用4-(4-甲基哌嗪-1-甲基)苯甲酰胺与N-(5-溴-2-甲苯基)-4-(3-吡啶基)嘧啶的C–N偶联反应描述了一种合成伊马替尼的新方法-2-胺形成伊马替尼。在这种合成路线中,纳米ZnO作为催化剂的高效率和高选择性是将4-(4-甲基哌嗪-1-甲基)苄腈温和水解为相应酰胺的关键。伊马替尼的总产率为51.3%,纯度为99.9%。这种简单有效的合成途径避免了基因杂质的产生(由FDA药物评估和研究中心分类),并且该合成对环境友好,反应时间短。
更新日期:2019-07-31
中文翻译:

伯酰胺与溴取代的嘧啶胺的CN偶联反应合成伊马替尼
通过使用4-(4-甲基哌嗪-1-甲基)苯甲酰胺与N-(5-溴-2-甲苯基)-4-(3-吡啶基)嘧啶的C–N偶联反应描述了一种合成伊马替尼的新方法-2-胺形成伊马替尼。在这种合成路线中,纳米ZnO作为催化剂的高效率和高选择性是将4-(4-甲基哌嗪-1-甲基)苄腈温和水解为相应酰胺的关键。伊马替尼的总产率为51.3%,纯度为99.9%。这种简单有效的合成途径避免了基因杂质的产生(由FDA药物评估和研究中心分类),并且该合成对环境友好,反应时间短。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号