当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterogeneous Catalytic Oxidation of Ammonia by Various Transition Metals
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2019-07-31 , DOI: 10.1021/acs.jchemed.9b00351 Petrus C. M. Laan 1 , Mareena C. Franke 1 , Richard van Lent 1 , Ludo B. F. Juurlink 1
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2019-07-31 , DOI: 10.1021/acs.jchemed.9b00351 Petrus C. M. Laan 1 , Mareena C. Franke 1 , Richard van Lent 1 , Ludo B. F. Juurlink 1
Affiliation
|
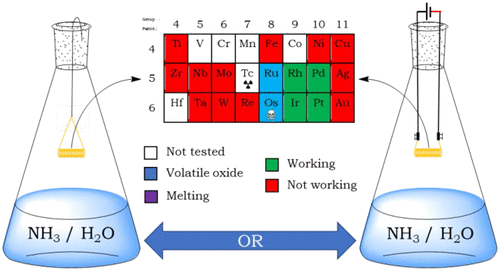
A well-known demonstration is adapted to simplify the illustration of heterogeneous catalytic oxidation of ammonia. Various metal catalyst wires are placed above the liquid level in a flask containing concentrated ammonia. After brief preheating, some metal wires continue to glow, providing visual evidence of an overall exothermic reaction taking place at the catalyst surface. Thermal heating by a butane flame prior to insertion and in situ resistive heating using a power supply yield identical results. Active catalysts are the group 9 and 10 elements Rh, Ir, Pd, and Pt. Besides the illustration of the Sabatier principle, the effect of the ammonia-to-oxygen ratio can also be visualized, and active metals vary in the production of a grayish smoke. These observations provide a starting point to discuss catalytic selectivity, a topic of great relevance to industrial catalytic oxidation of ammonia.
中文翻译:

各种过渡金属对氨的非均相催化氧化
众所周知的演示适于简化氨的非均相催化氧化的图示。将各种金属催化剂丝放在液位上方的装有浓氨水的烧瓶中。经过短暂的预热后,一些金属丝继续发光,从而提供了可见的证据,表明在催化剂表面发生了总体放热反应。在插入前和原位通过丁烷火焰进行热加热使用电源进行电阻加热可获得相同的结果。活性催化剂是第9和10组元素Rh,Ir,Pd和Pt。除了说明Sabatier原理外,还可以观察到氨氧比的影响,并且活性金属在发灰烟气中也会发生变化。这些观察结果为讨论催化选择性提供了一个起点,催化选择性是与氨的工业催化氧化高度相关的话题。
更新日期:2019-08-01
中文翻译:

各种过渡金属对氨的非均相催化氧化
众所周知的演示适于简化氨的非均相催化氧化的图示。将各种金属催化剂丝放在液位上方的装有浓氨水的烧瓶中。经过短暂的预热后,一些金属丝继续发光,从而提供了可见的证据,表明在催化剂表面发生了总体放热反应。在插入前和原位通过丁烷火焰进行热加热使用电源进行电阻加热可获得相同的结果。活性催化剂是第9和10组元素Rh,Ir,Pd和Pt。除了说明Sabatier原理外,还可以观察到氨氧比的影响,并且活性金属在发灰烟气中也会发生变化。这些观察结果为讨论催化选择性提供了一个起点,催化选择性是与氨的工业催化氧化高度相关的话题。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号