当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Dearomatization: Evolution from Chemicals to Traceless Electrons
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-10-10 , DOI: 10.1002/adsc.201900750 Shide Lv 1 , Guofeng Zhang 1 , Jianbin Chen 1 , Wei Gao 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-10-10 , DOI: 10.1002/adsc.201900750 Shide Lv 1 , Guofeng Zhang 1 , Jianbin Chen 1 , Wei Gao 1
Affiliation
|
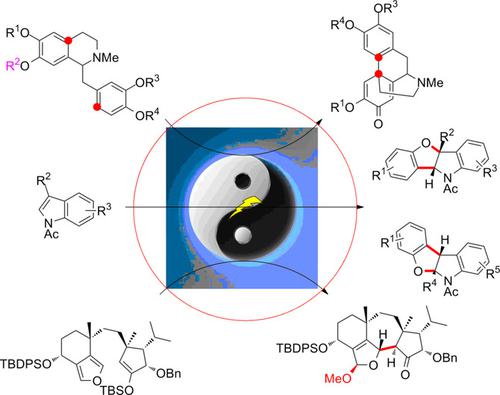
Dearomatization reactions represent a versatile approach for the preparation of three‐dimensionally (3D) privileged cyclic moieties from simple planar aromatic compounds. However, exogeneous oxidants are required for most of the radical and oxidative dearomatizations. Therefore, sustainable procedures are in high demand, especially those in the absence of external oxidizing reagents. Fortunately, electrolytic dearomatization protocols can fulfill the above requirements due to the manipulation of traceless electrons instead of chemicals during the processes. Nevertheless, sustainable electrochemical dearomative transformations have been far less frequently investigated than the well‐developed chemical dearomatization reactions. Herein, we summarize representative breakthroughs in the electrochemical dearomative transformation of indoles, furans and activated arenes (phenols and anisoles) for the synthesis of complicated skeletons. Hopefully, this interesting “simplicity‐to‐complexity” synthetic logic will inspire more innovations from the electroorganic community.
中文翻译:

电化学脱芳香化:从化学到无痕电子的演变
脱芳香化反应代表了一种从简单的平面芳族化合物制备三维(3D)优先环状部分的通用方法。但是,大多数自由基和氧化脱芳烃反应都需要外源性氧化剂。因此,迫切需要可持续的程序,尤其是在没有外部氧化剂的情况下。幸运的是,由于在处理过程中操纵了无痕电子而不是化学物质,因此电解脱芳香化方案可以满足上述要求。然而,与发达的化学脱芳香化反应相比,可持续的电化学脱芳香化转变的研究频率要低得多。在这里,我们总结了吲哚的电化学脱芳香转化中的代表性突破,呋喃和活化的芳烃(苯酚和茴香醚),用于合成复杂的骨架。希望这种有趣的“从简单到复杂”的合成逻辑将激发有机电子界的更多创新。
更新日期:2019-10-10
中文翻译:

电化学脱芳香化:从化学到无痕电子的演变
脱芳香化反应代表了一种从简单的平面芳族化合物制备三维(3D)优先环状部分的通用方法。但是,大多数自由基和氧化脱芳烃反应都需要外源性氧化剂。因此,迫切需要可持续的程序,尤其是在没有外部氧化剂的情况下。幸运的是,由于在处理过程中操纵了无痕电子而不是化学物质,因此电解脱芳香化方案可以满足上述要求。然而,与发达的化学脱芳香化反应相比,可持续的电化学脱芳香化转变的研究频率要低得多。在这里,我们总结了吲哚的电化学脱芳香转化中的代表性突破,呋喃和活化的芳烃(苯酚和茴香醚),用于合成复杂的骨架。希望这种有趣的“从简单到复杂”的合成逻辑将激发有机电子界的更多创新。































 京公网安备 11010802027423号
京公网安备 11010802027423号